20240119-Protolabs Insight - What is Anodising?
Anodising produces aluminuim oxide on the surface of the metal, helping to protect your part against abrasion and corrosion. This video talks about anodising and why you may want to use the service for your metal parts.
The Protolabs Insight video series will help you master digital manufacturing, covering cover specific topics such as choosing the right 3D printing material, optimising your design for CNC machining, surface finishes for moulded parts, and much more.
Insight:
Transcript
Hi and welcome to this week’s Insight video for Friday.
Just to remind you that these videos are all about giving you some practical advice to help you design better parts for digital manufacturing. Whether that part is produced by 3D printing, injection moulding or CNC machining, we want to help you get it right.
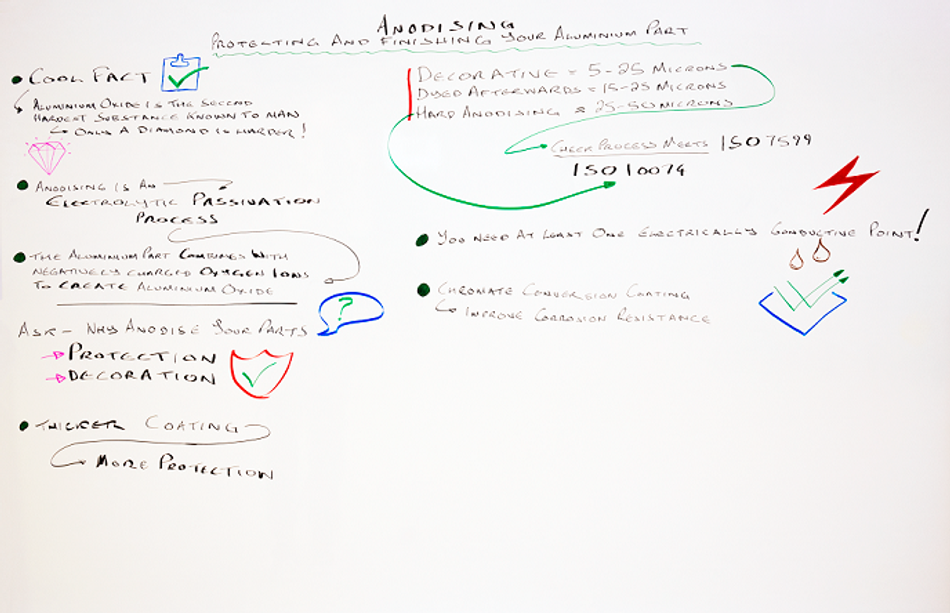
This week we are taking a closer look at anodising aluminium parts and why it is a great way to protect this metal or to add a decorative finish.
Let’s start with a really cool fact. Anodising produces aluminium oxide on the surface of the metal. Don’t worry that’s not the cool fact.
Here it comes. Aluminium oxide is the second hardest substance known to man, only a diamond is harder. I’ll give you a second for that to sink in.
This means that anodising can protect your part against abrasion and also corrosion. It’s also good to look at and can be dyed, so you may opt for it for purely aesthetic reasons.
In the next couple of minutes, we are going to explore the process of anodising, but more importantly I will help you get what you want from it.
But first of all, here’s the science bit.
Anodising is an electrolytic passivation process. It’s called anodising because the part to be treated forms the anode electrode of an electrical circuit.
The most common method is sulphuric acid anodising. The part is cleaned, placed in the sulphuric acid and then a positive charge is applied to it and a negative charge is applied to plates that are also immersed in the acid. The aluminium part combines with the negatively charged oxygen ions to create aluminum oxide.
Okay, science lesson over. Let’s move onto what you really need to know.
The first question that you should ask yourself is why do you want the part anodised? Is it because you need to protect the part? Or is it because you want to achieve a decorative finish?
Your answer to this question will affect the thickness of the coating you need. The thicker the coating, the more protection your part will have.
Now we are only talking about microns here or millionths of a metre, but as the saying goes: every little bit counts.
For decorative finishes you will need a minimum thickness of between 5 and 25 microns. If you want the part dyed afterwards, then we would recommend something between 15 and 25 microns.
Of course, a thicker layer will improve the part’s abrasion and corrosion resistance. If it needs this protection, then it should have what we call a hard anodising finish. This produces a thickness of between 25 and 50 microns.
Let’s say that you want your part anodising. How do you know that your supplier is up to scratch? Well here’s a couple of standards for you. For decorative anodising check that the process meets ISO 7599. And for hard anodising it needs to meet ISO 10074.
Okay, what else do you need to think about?
Well you remember that we talked about how your aluminum part forms part of an electrical circuit? This means that it needs to have at least one electrically conductive point in the component at all times. That point will get no coverage and will not be anodised.
Now don’t worry too much because we are probably only talking about 0.1% of the surface on your part.
What it does mean, is that you will need to think about how your component will be mounted or jigged. Jigging serves two purposes, it maintains the electrical contact and it also ensures that the part does not fall off during the process. And we don’t want that to happen do we?
This conducting or jigging point is often a threaded hole which gives a great electrical contact.
It needs to be even more secure for hard anodising because this process needs higher voltages, so your supplier may need to tap a threaded bolt.
Our best advice is for you to work with your supplier. Typically the best place would be on a hidden face or a non-critical surface to hide the contact marks.
So anodising is a great choice for protecting aluminum parts, but as they say on the BBC, other options are available to you.
It really comes down to your design needs and what functionality you need.
For instance another good option is chromate conversion coating. It provides a protective film to improve corrosion resistance for your aluminium part and is a great primer for paint. It also retains the metal’s conductivity.
So, there you have it. What is anodising? - Anodising is great if you want a decorative finish or to protect your aluminium part. It is not the only solution though, so don’t get sold on the idea if your supplier only provides this and not alternatives.
That’s it for this week. I hope you found the video informative.
Next time I’ll be taking a closer look at prototyping small parts and how a new material could help you with this.
Until next week.
Watch other Protolabs Insight videos here.