3D Bioprinting Gives Researchers the Power To Create Artificial Organs
In the near future, 3D bioprinting will allow medical professionals to engineer and 3D print patient-specific organs for organ transplants in a few hours.
Israeli researchers create the first 3D printed heart. Source: Israel 21
The evolving versatility of three-dimensional (3D) printing continues to accelerate its mass adoption across industries. The disruptive power of additive manufacturing AM has helped facilitate the industry's billion-dollar growth. By 2026, the 3D printing market industry size is scheduled to reach $51.77 billion (EXTERNAL).
Enterprises within industries like aerospace & defense and the automotive have fully embraced AM, using it to manufacture cost-effective rapid prototypes and functional, complex low-production parts. However, one sector that occasionally gets overlooked when discussing 3D printing is medicine. Additive manufacturing has led to remarkable advances in the healthcare sector, with some of the most exciting advances happening just this past year. The exceedingly rising demand for organ transplants and advancements in biomaterial development (BAD)have given rise to a new additive manufacturing technique.
Medical researchers can "print" cells, tissues, and organs on demand.
Dubbed 3D bioprinting, this new medicine-specific process combines biomaterials like cells and growth factors to create tissue-like structures that imitate natural tissues. At its core, 3D bioprinting works very similarly to other AM techniques used in other industries and on your desktop. Researchers take a digital model and print their desired object layer by layer.
Nonetheless, scientists frequently use a living cell suspension rather than common 3D printing materials like resin or thermoplastic (EXTERNAL). Biomaterial parameters like biocompatibility, cell viability, and cellular microenvironment impact the final printed product.
Bioprinting can be used to engineer artificial organs.
The limited availability of organs globally plays a crucial role in advancing 3D bioprinting. The ability to print-on-demand lost or failed organs and tissues for affected patients would be revolutionary. Secondly, in clinical practice, bioprinted models are increasingly used for surgical planning, pharmaceutical testing, and medical education.
Start-ups and researchers worldwide are currently investigating the disruptive power of tissue engineering and artificial organs, with clinical trials on the horizon. These institutions have printed various tissues like vasculature, hearts (BAD), bones, cartilage, skin, and livers.
This article review on bioprinting will present key aspects of this additive manufacturing technique, applications, recent advances, and the multidisciplinary research transforming bioprinting.
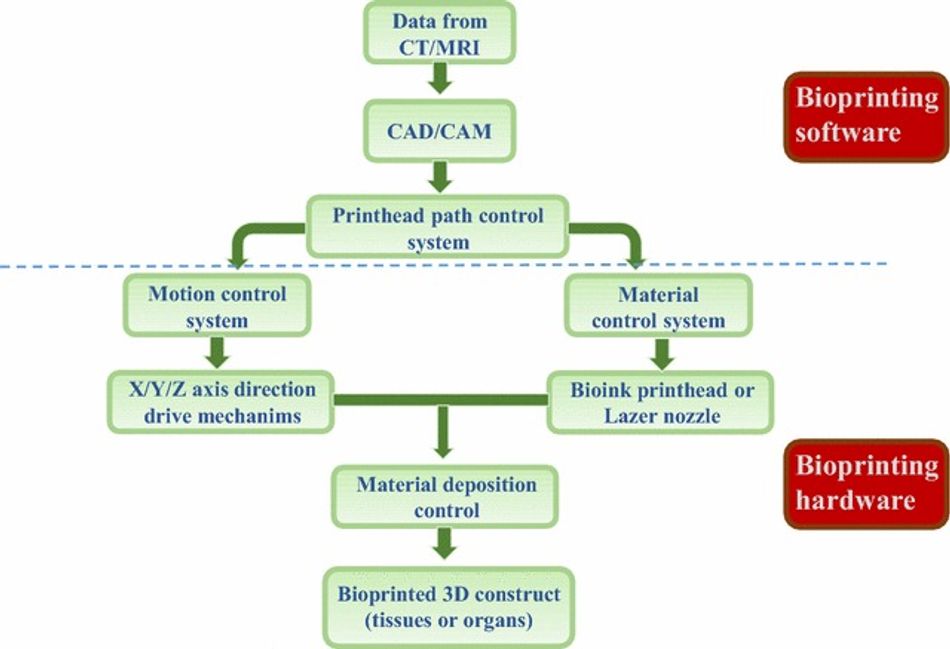
Bioprinting shares some similarities to traditional additive manufacturing techniques, but it is far more complicated. 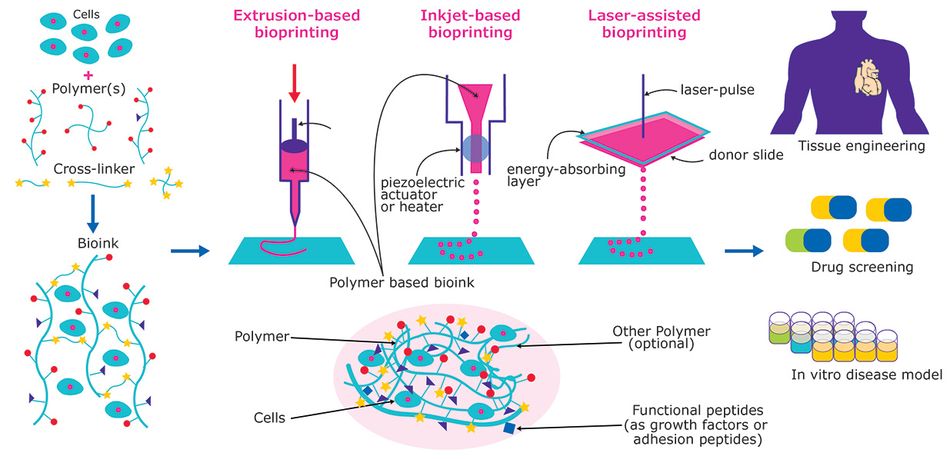 The bioprinting process. Source: Material Matters/Merck (EXTERNAL)
The bioprinting process. Source: Material Matters/Merck (EXTERNAL)

This additive fabrication method uses cells and other biocompatible materials, known as bio-ink, to print living structures layer by layer. Most of the bioprinting methods (EXTERNAL) currently available are based on either laser, acoustic, inkjet, or extrusion technologies. More or less, all of the methods use a standard series of steps to fabricate living tissue. Bioprinting involves preparation, printing, maturation, and application.
It all starts with 3D imaging. Like any other additive manufacturing technique, you need a 3D file or blueprint for your intended creation. However, in the case of bioprinting, this model usually comes from a Magnetic Resonance Imaging (EXTERNAL)(MRI) scan or a Computed Tomography (CT) scan. There are cases where bioprints were fabricated using a Computer-aided design (CAD) program or a file downloaded from the internet.
Next, your model is prepared for the printer using software. Your 3D model from the previous step is converted to an STL (EXTERNAL); then, this STL file is converted to a gcode (EXTERNAL)file. The blueprint includes layer-by-layer instructions in great detail. Here, researchers can optimize their print parameters on a microscopic level, such as cell viability, as well as macroscopic outcomes, such as pore size and structural fidelity.
Subsequently, during the preparation stage, researchers select a cell type. The type of cell or cell line will determine many of the constraints of a project. Researchers have to find a balance between the clinical relevance and how easy it is to work with the selected cells— no easy task. For example, nerve tissue is acutely complex while other tissues like tendons and skins are much more scientifically understood, making them much easier to cultivate.
Here researchers must consider factors like the type of bio-ink needed for the project. For the uninitiated, a bio-ink (BAD) is a combination of living cells and a compatible base. This compatible base might comprise of biomaterials like collagen, gelatin, hyaluronan, silk, alginate, or nanocellulose (EXTERNAL). In short, this biomaterial offers cell scaffolding to grow on and nutrients to survive on. We will discuss bio-ink in greater detail later.
Finally, the object is printed. The bioengineering printing process involves depositing your selected bio-ink layer by layer. Here fidelity layers are no more than 0.5mm. The printer's nozzle length, profile, and diameter directly impact the pressures, temperature, and speeds you can print. Additionally, pairing the right bio-ink with the right nozzle is something scientists consider. Regardless, our print comes out as a highly viscous fluid, forming the model.
The print is solidified. As the pattern fabricates layer by layer, this viscous liquid begins to harden, thanks to the process of crosslinking. Crosslinking is a procedure by which the molecules of a soft network change their interactions, causing the material to stiffen. This process can usually be aided with UV light chemicals or heat.
In one application, these newly created 3D structures can be used by researchers to conduct in vitro studies better than their 2D counterparts. This is because a 3D bioprinted structure's geometry is more similar to that of a naturally occurring biological system, making it more biologically relevant. Overall, this 3D printing technology is used commonly in tissue engineering, bioengineering, and material science (GOOD).
Tissue engineering starts with bio-inks.
Bio-inks are an essential part of bioprinting. If anything, you can describe them as the filament for this specific fabrication method. These "carriers", natural or synthetic, mimic the Extracellular Matrix (EXTERNAL) (ECM) to support the adhesion, proliferation, and differentiation of living cells. In short, bio-inks give cells what they need to live, grow, and create functional 3D tissue (EXTERNAL).
Though it is possible to have bio-inks composed of just cells, in most cases, an additional carrier material is included in the bio-ink. Enveloping the cells used for the bioprint, this additional material is usually a biopolymer soup that acts as a 3D molecular scaffold. In a nutshell, they protect and preserve the cells during the printing process. The right mixture of biopolymers helps medical scientists achieve the perfect balance of chemical, physical, and biological constraints.
There is a wide range of bio-ink polymers available to researchers. They are separated into three categories, matrix ink, support ink, and sacrificial ink.
Matrix bio-ink polymers hold and support cells during the bioprint process. Two commonly used matrix bio-ink polymers are collagen and alginate (EXTERNAL). Thanks to its sound viscoelastic properties, its agglutinative nature with cells, and ability to improve the mechanical stability of tissues, collagen (usually synthetic) is a commonly used polymer. In comparison, polymers like alginate are used to provide water and nutrients to cells from the surrounding medium.
Support inks polymers are biocompatible materials that offer better mechanical properties than matrix inks. As the name implies, these matrix inks support bioprint constructs where hydrogels fall flat (BAD). They are commonly used for connective or hard tissue like the bio-fabrication of cartilage, bone, or muscle tissues.
Sacrificial inks polymers are designed to provide temporary support during the creation of complex negative geometries like vascular networks (EXTERNAL). They are usually removed in post-processing.
Aside from soups of bio-ink polymers, other biomaterials like support baths and additives are used to create microenvironments that mimic biological systems. To print a geometrically complex structure, researchers may use a support method known as Freeform Reversible Embedding of Suspended Hydrogels (EXTERNAL) (FRESH). This bioprinting method allows for the fabrication of soft cell encapsulated materials like collagen or alginate. Simultaneously, to help regulate cell behaviors such as adhesion, migration, and proliferation, scientists might use protein additives.
Researchers have a wide range of natural and synthetic materials at their disposal. The biggest challenge is navigating and balancing the properties of these biomaterials. The continuous research into the realms of bioprinting is expanding the market of bio-ink, giving scientists a plethora of options.
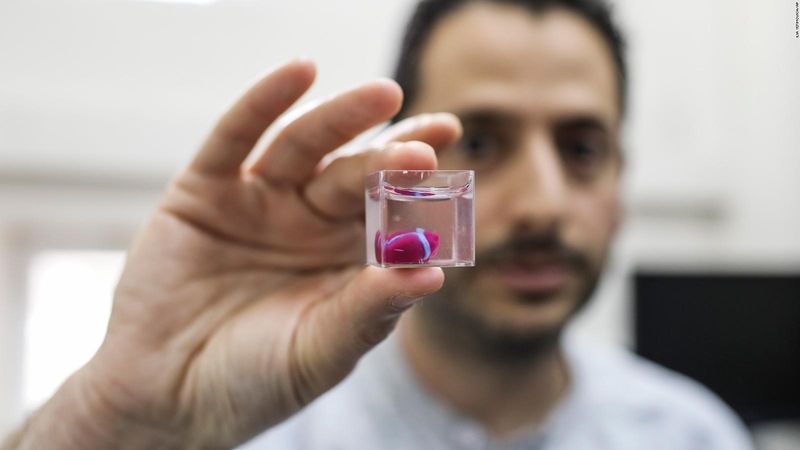
Researchers printed a thumb-sized heart in 2019.
In 2019, for the first time, researchers from the University of Tel Aviv (EXTERNAL) 3D printed a small vascularized heart. The team of scientists created a bio-ink cocktail derived from fatty tissue from both humans and pigs to print the heart.
The tissue's cellular materials were separated while the cells were genetically "reprogrammed" to become pluripotent stem cells. Pluripotent stem cells are a handy tool when printing living tissues. These "master cells" can make cells from all three basic body layers, so they can potentially produce any cell or tissue the body needs to repair itself.
All of the materials were mixed together with a thermoresponsive hydrogel that becomes stronger upon heating to the human body's temperature. After creating an anatomical model using a CT scan, researchers fed the bio-ink mixture of hydrogel and stem cells into a 3D printer. They were able to print a thumb-size heart in three hours. The engineered heart featured cells, blood vessels, ventricles, and chambers. Though the team is still figuring out how to coax it to beat and circulate blood, the project marks a vital bioprinting milestone.
"Maybe, in ten years, there will be organ printers in the finest hospitals around the world, and these procedures will be conducted routinely, says (EXTERNAL) the University of Tel Aviv team. Here like in many of our next examples, bioprinting demonstrates its revolutionary potential.
According to the University of San Francisco School of Health, (EXTERNAL) a patient on the waiting list for a heart transplant can end up waiting a minimum of six months, with a majority of cases taking significantly longer. Bioprinting a heart puts us on the path towards a faster alternative solution.
3D printed corneas could be used to treat corneal blindness.

Millions of people around the world have corneal blindness due to disease or scarring. The cornea acts as the outermost lens of the eye. It can be described as the window that controls and focuses the entry of light into the eye. In short, the cornea contributes between 65-75% of the eye's total focusing power.
To fix corneal blindness, a patient usually needs a cornea transplant. However, rather than wait on donated eye tissue, Newcastle University researchers have come up with a potential replacement. In 2018, the Newcastle University team 3D printed a human cornea for the first time. Utilizing a bio-ink mixture of stem cells (BAD), alginate, and collage, they were able to print a cornea in less than 10 minutes.
Building off this study, a research team from Marmara University in Turkey has developed a 3D printed cornea potentially suitable for transplantation (EXTERNAL) in 2020. This would allow for livers to effectively match this patient's immunological, cellular, and anatomical properties.
Patient-specific bioprinted livers could become a reality.
In 2016, researchers from the University of California San Diego managed to bio-fabricate liver tissues that looked and functioned like real liver structures. Though initially used by the pharmaceutical industry for drug development and testing, medical researchers have become more ambitious.
In 2018, the medical technology company Organovo (EXTERNAL) bioprinted liver tissue patches and implanted them into living mice with positive results. The liver tissue functioned normally in the mice with no issues. Human trials are expected to begin within the next year. Finally, Brazilian researchers from the University of São Paulo have reported they are exploring printing livers with human cells and patient-specific biological materials.
Bioprinted skin could be used to treat burn victims.
This past year researchers from the University of Toronto Faculty of Applied Science & Engineering developed a novel new handheld device that can 3D print skin (EXTERNAL). The first device of its kind, the 3D skin-roller printer can deposit sheets of skin stripe by stripe, covering large burn wounds. At the same time, its optimized bio-ink can accelerate the healing process. The bio-ink dispensed by the roller is composed of mesenchymal stroma cells.
These stem cells promote skin regeneration and reduce scarring. The printer forms in sintu, depositing, and setting in place in two minutes or less. The printer could be a potential tool for treating burn victims.
"Once it's used in an operating room, I think this printer will be a game-changer in saving lives. With a device like this, it could change the entirety of how we practice burn and trauma care", says the research team.
Why is bioprinting important?
There are a lot of benefits to bioprinting. Logically, tissue-like structures that mimic the actual micro-and macro-environment of human tissues and organs offer researchers a host of opportunities in the medical community. The nascent technology is becoming essential in clinical trials and drug testing. Other growing areas like artificial organs, bone tissue regeneration, and cosmetic surgery are all currently being explored.
Nevertheless, researchers still have a ways to go. 3D bioprinting is still falling short. Human-specific tissues are difficult to fabricate due to the complexities in tissue-specific extracellular matrices. There is also an absence of a suitable co-culture medium to support multiple types of cells. In short, researchers need to find a way to optimize the tissue maturation process. There is hope.
Bioprinting is clearly evolving and could start coming into maturity in the coming years. Where do you think the technology will be in the next decade?