SPT Vilecon Accelerates Medical Device Development with Freeform Injection Molding
Medical device manufacturer adopts Nexa3D’s FIM process to shorten times-to-market on complex medical devices
This article was first published on
nexa3d.comSPT Vilecon is a fully integrated provider of development and manufacturing services to the medical device industry, working at the intersection of design, development, and manufacturing to allow customers to move a new product from concept to commercialization, and with inhouse tool building in place to ensure shortest possible lead-times. Certified according to ISO 13485, the European standard governing medical device manufacturing, they provide product development support as well as injection molding and other manufacturing services to a large number of companies in northern Europe.
Recognizing that speed is always of the essence, SPT decided to implement Nexa3D Freeform Injection Molding. The goal was to help customers shorten times-to-market on complex medical devices in demanding medical-grade materials and to expand on the range of materials that can be quickly and reliably used in prototyping and early device test manufacturing.
The challenge
Medical device development requires production-grade prototypes
Medical device manufacturers face rigorous regulatory requirements when introducing new devices into the market. A medical device needs to be safe and efficient to use for the intended purpose, and it is the job of the manufacturer to demonstrate that a given device meets these essential requirements. To do so, manufacturers must develop elaborate test protocols that evaluate every aspect of device manufacturing and usage, and they must ensure that tests are carried out in a way that accurately represents the conditions that apply for the manufacturing and usage of the finished medical device. The later these tests are carried out, the more severe the consequences are if a test fails. Medical device companies are constantly on the lookout for tools and methods that help them improve early-stage device testing to reduce development risks and costs of rework.
SPT Vilecon often works with Silicones, a group of materials that are heavily used in the medical device industry due to their excellent chemical resistance, mechanical performance and biocompatibility. However, silicone parts are notoriously difficult to prototype and test, since most grades need to be injection-molded to achieve their full performance.
“Freeform Injection Molding has enabled us to dramatically accelerate the delivery of production-grade prototypes and low-volume productions in challenging materials and designs.”– Bjarne Andersen, CEO, STP Vilecon
The solution
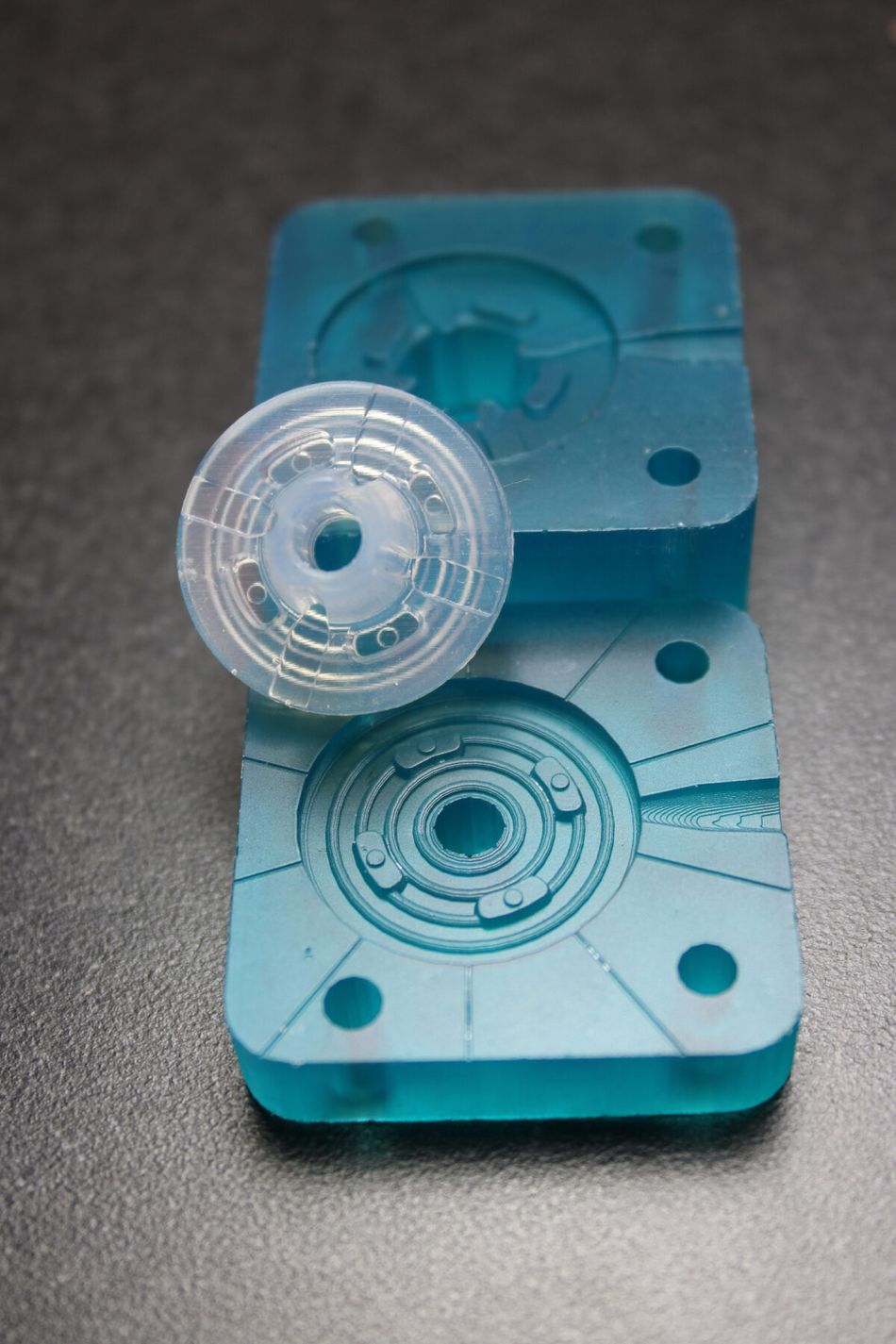
3D printed injection mold tooling allows early verification, new applications
To meet customer demands for rapid prototyping of silicones, and other complex medical-grade materials, SPT Vilecon wanted a prototyping platform that would integrate seamlessly with their existing tool-building and injection molding capabilities. Other key requirements included biocompatibility, compatibility with the wide range of thermoplastics and silicones already in use, as well as design freedom for complex part prototyping. In the end, they decided to implement Nexa3D’s XiP desktop system and Freeform Injection Molding with xMOLD resin. This compact and highly versatile platform was the only alternative that met all the key requirements, while at the same time enabling SPT Vilecon to expand into new domains based on metal and ceramic injection molding.
The benefits
2-day development and verification of complex silicone parts
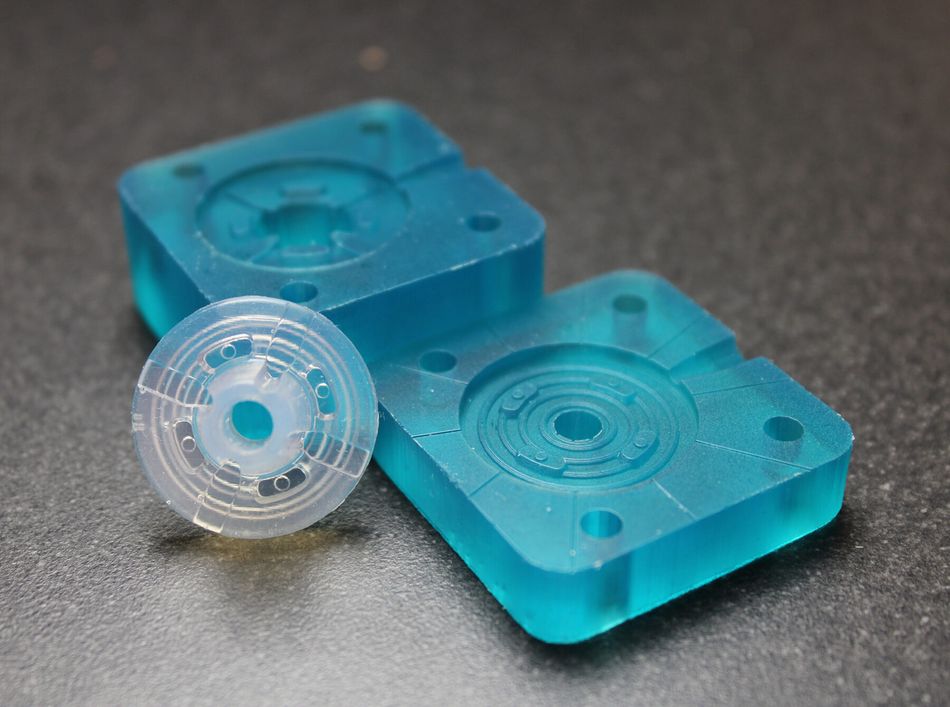
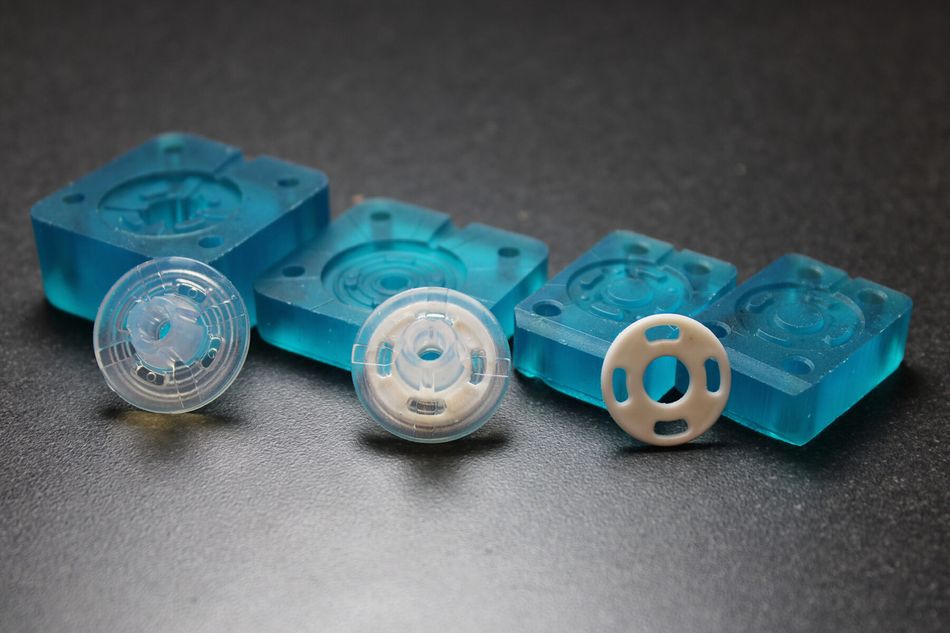
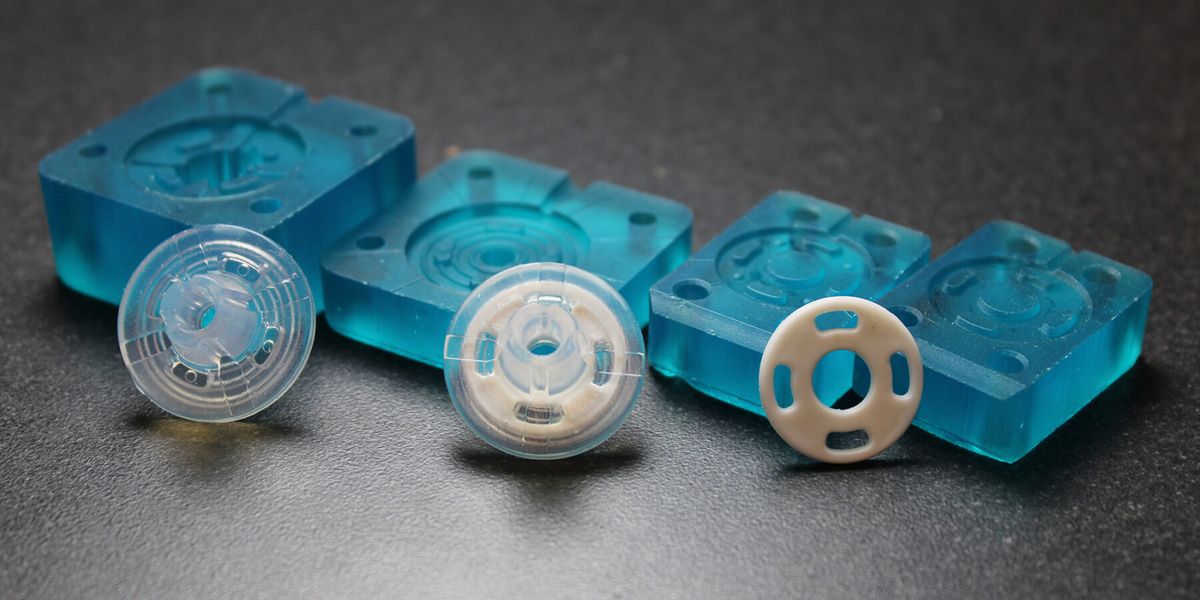
With Freeform Injection Molding, SPT Vilecon was able to offer a customer developing an intravenous silicone product, early design and material verification by utilization of 3D printed tools.
Early Verification: The use of 3D-printed xMOLD tooling enabled the customer to obtain injection-molded silicone parts in a medical-grade silicone within two days, and to verify key design and performance aspects.
Rapid iterations: At the same time, important design input was collected from the first tests, which could be integrated in a second iteration that took only a couple of hours to complete. In comparison, the conventional metal tooling that would normally be needed for this kind of verification would have taken more than six weeks to design and procure, and the adjustments needed for the second iteration would have taken the inhouse tool-shop a week to complete.
Cost and time savings: The costs of the 3D-printed silicone molds for the first and second iterations were less than EUR 2.000 combined. And the two-day design cycle was more than 90% faster than the speed achievable with conventional metal tooling.
Seamless scalability: Based on the design input collected from the first two iterations, the design of the silicone part was approved and the manufacturing of a metal tool could be initiated with full confidence of part moldability and performance. The collected design input and learnings enabled the inhouse tool-shop to produce the metal tooling within 4 weeks, with no iterations needed in the final tool.
Customer Impact
Compliance with project timeline, budget ensured through early verification
Medical device manufacturers face strong pressures to bring new and innovative products to market. Robust and valid prototyping and verification is key to minimize costs, time and risks associated with the development. By implementing Freeform Injection Molding, SPT Vilecon has created a new toolbox for medical device companies seeking to accelerate their innovation and obtain early verification of part performance and moldability.
Using the XiP with Freeform Injection Molding, a medical device manufacturer was able to save 6 weeks, and 87% of prototyping investments, while at the same time removing a challenging silicone component from the critical path of product development.
SPT Vilecon has been able to start expanding the range of injection mold prototyping into the domains of silicone, metal and ceramic injection molding, to provide medical device manufacturers with an even wider selection of product development and verification services.