Wearable sensors for Parkinson's can improve with machine learning, data from healthy adults
Low-cost, wearable sensors could increase access to care for patients with Parkinson’s disease. New machine-learning approaches and a baseline of data from healthy older adults improve the accuracy of the results from such sensors

Illinois professors Richard Sowers, left, and Manuel Hernandez used machine learning techniques to improve data analysis from wearable sensors to detect symptoms and monitor the progression of Parkinson’s disease. Photo by Fred Zwicky
This article was first published on
news.illinois.eduLow-cost, wearable sensors could increase access to care for patients with Parkinson’s disease. New machine-learning approaches and a baseline of data from healthy older adults improve the accuracy of the results from such sensors, University of Illinois Urbana-Champaign researchers and clinical collaborators found in a new study.
“This study demonstrates that the expansion of datasets with healthy older adult motion data and integration with deep learning approaches can help improve the accuracy of detecting differences in motor impairment in persons with Parkinson's disease for use in future telemedicine sessions,” said study leader Manuel Enrique Hernandez, a professor of biomedical and translational sciences within the Carle Illinois College of Medicine at the U. of I.
To have their symptoms monitored, patients with Parkinson’s disease must periodically undergo assessment — typically a time-consuming, in-person visit to a specialist with limited availability, Hernandez said.
Evaluation by telemedicine would improve access to care for patients but is made challenging by the lack of quantifiable measurements. For example, the physician can watch the patient move, but cannot assess rigidity or muscle tone. While some progress has been made in using wearable sensors to assess motor skills, the cost limits their use.
To address these challenges, the Illinois team focused on ways to improve assessment using low-cost wearable sensors.
“Ideally, we’d have something that’s completely passive, gathering data as a person goes through their everyday environment and using that information to provide guidance on their overall motor function and progression of neurological symptoms. But then you face the big challenge of how to parse all that information and make it useful to a clinician,” Hernandez said. “That’s what led to our strategy of improving the machine learning, honing in on activities useful for assessment and looking at healthy individuals as a baseline.”
The researchers adapted the gold standard of clinical assessment, the Movement Disorders Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale. The MDS-UPDRS outlines specific tasks that a patient would perform and qualitative observations a physician would make during an exam and organizes them into categories for scoring.
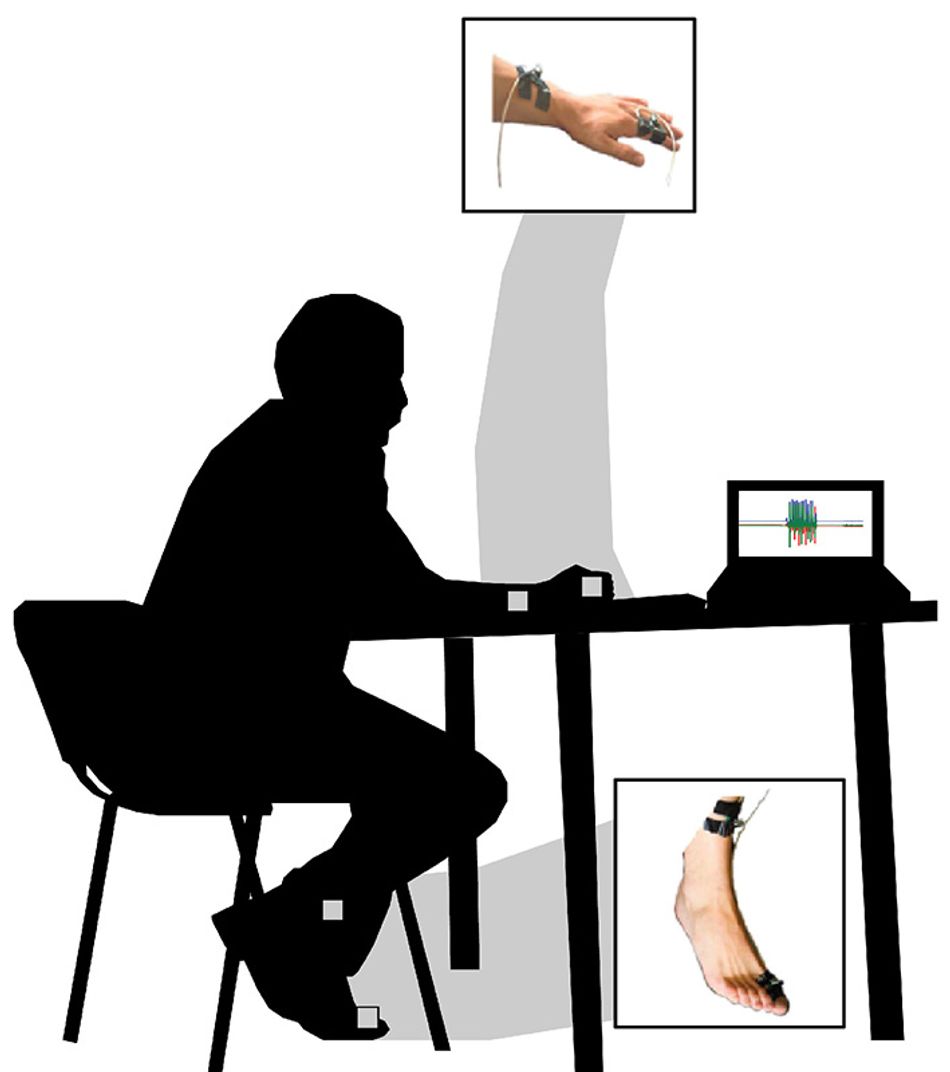
Subjects completed tasks normally done during in-person clinical assessment with sensors on their arms, hands and feet to gather data. Image courtesy of Manuel Enrique Hernandez
For their study, the researchers had patients perform tasks and muscle movements while wearing sensors to provide the data in the categories scored by MDS-UPDRS. They used data from both patients with Parkinson’s disease and from healthy older adults to train their machine learning model.
“Improving machine learning models requires a lot of data. We hypothesized that healthy individuals can serve as the basis for what to expect with potential age-related changes. Then we could build a comparative model to get a sense of when there might be a marked change in terms of motor function or motor impairment,” said Hernandez, who also is affiliated with the departments of kinesiology and community health and bioengineering, as well as the Beckman Institute for Advanced Science and Technology at Illinois.
The researchers also used deep learning techniques known as pretraining to make the model more robust, better able to identify important data points and filter out irrelevant ones. The pretraining and the addition of healthy older adult data improved the accuracy of identifying motor impairments in hand movement tasks by more than 12% over the current standard model. Additionally, including data from healthy older adults improved the accuracy of assessing every task for the upper and lower body, except for toe tapping.
The results were reported in the journal Sensors.
Next, the researchers hope to collaborate further with neurologists and clinicians to expand their model. They aim to identify a small number of additional tasks that could quantitatively measure more information about the classical symptoms of Parkinson’s disease, such as tremor, while maintaining low cost and ease of use for the sensors.
“There’s a very large need for us to better understand and better quantify changes that are ongoing with Parkinson’s disease,” Hernandez said. “The ability to use wearable sensors while performing activities that are part of the course of clinical evaluations in a telehealth setting might open up the doors for more objective, more quantifiable information that can help direct treatment for individuals with Parkinson’s disease and hopefully improve quality of life moving forward.”
Richard Sowers, Illinois professor of industrial and enterprise systems engineering and mathematics, and James R. Brasic, professor and neurologist at Johns Hopkins School of Medicine, New York University and NYC Health + Hospitals, were coauthors of the paper.
Editor’s note:
To reach Manuel Enrique Hernandez, email mhernand@illinois.edu.
The paper, “A deep learning approach for automatic and objective grading of the motor impairment severity in Parkinson’s disease for use in tele-assessments,” is available online. DOI: 10.3390/s23219004
This research had no external funding.