Diving into Organoid Intelligence
Organoid intelligence is a pioneering field where scientists use human brain cells to improve computer functions.

Engineers at UC San Diego demonstrated for the first time that human brain organoids implanted in mice have established functional connectivity to the animals’ cortex. Picture by: David Baillot | UC San Diego Jacobs School of Engineering | Flickr
Introduction
The field of organoid intelligence is recognized as groundbreaking. In this field, scientists utilize human brain cells to enhance computer functionality. They cultivate tissues in laboratories that mimic real organs, particularly the brain. These brain organoids can perform brain-like functions and are being developed by Dr. Thomas Hartung and his team at the Johns Hopkins Bloomberg School of Public Health.
For nearly two decades, scientists have used organoids to conduct experiments without harming humans or animals. Hartung, who has been cultivating brain organoids from human skin samples since 2012, aims to integrate these organoids into computing. This approach promises more energy-efficient computing than current supercomputers and could revolutionize drug testing, improve our understanding of the human brain, and push the boundaries of computing technology.
The conducted research highlights the potential of biocomputing to surpass the limitations of traditional computing and AI. Despite AI's advancements, it still falls short of replicating the human brain's capabilities, such as energy efficiency, learning, and complex decision-making. The human brain's capacity for information storage and energy efficiency remains unparalleled by modern computers. Hartung's work with brain organoids, inspired by Nobel Prize-winning stem cell research, aims to replicate cognitive functions in the lab. This research could open new avenues for understanding the human brain by allowing ethical experimentation. The team envisions scaling up the size of brain organoids and developing communication tools for input and output, enabling more complex tasks.
A significant innovation by Hartung's team is a brain-computer interface device, resembling an EEG cap for organoids, facilitating signal transmission to and from the brain organoids. This development lays the groundwork for a synergistic relationship between AI and organoid intelligence, exploring each other's capabilities and potentially leading to technological advancements previously unimagined.
Understanding Organoids
Brain organoids are tiny, lab-created clusters of brain cells that simulate the brain's structure and function. Starting from skin cells, scientists reprogram these cells into stem cells and eventually into brain cells, creating organoids around 500 micrometers in size with fewer than 100,000 cells. Although these organoids are much smaller than a human brain, they offer a model for studying brain functions and developing computing systems.
Organoid-based bio computers have several advantages over traditional silicon computers. While computers excel at number crunching, the human brain is far superior in handling complex computations efficiently, such as learning to differentiate objects with minimal data. For example, the AI system AlphaGo required training on 160,000 game scenarios to master Go, something far beyond human capability in terms of time and energy consumption. Moreover, the brain's energy efficiency and data storage capabilities are unmatched by current technology, with an estimated storage capacity of 2,500 terabytes.
Despite not being miniature brains, brain organoids share important functional and structural characteristics with the human brain, suggesting their potential to achieve similar computational abilities. A study showing neurons in a dish learning to play Pong illustrates this potential. However, for organoid intelligence to be fully realized, brain organoids need to be larger and more complex, with millions of cells and a diversity of cell types that are important for learning and memory.
Despite not being miniature brains, brain organoids share important functional and structural characteristics with the human brain, suggesting their potential to achieve similar computational abilities. A study showing neurons in a dish learning to play Pong illustrates this potential. However, for organoid intelligence to be fully realized, brain organoids need to be larger and more complex, with millions of cells and a diversity of cell types that are important for learning and memory.
Improvements in brain organoid technology are essential for advancing organoid intelligence that includes increasing the size and cell diversity of organoids and improving their ability to express genes that improve learning and memory. Additionally, developing a way to nourish larger organoids, potentially through artificial blood vessels created by microfluidic technology, is considered to be challenging task. This technology would not only support the organoids' health but also enable communication through chemical signals, enabling more sophisticated biological computing systems.
Mechanisms of Organoid Intelligence
The integration of stem cell biology, bioengineering, and AI is forming novel approaches in the field of organoid research. This synergy is maximizing development of the stem cell, precision engineering techniques, and the analytical advantage of AI to transform the comprehension of how organisms develop, how diseases arise, and how we can devise new treatment methods. Machine learning algorithms have confirmed how closely organoids resemble actual human organs by analysing complex data sets, such as comparing organoid RNA sequencing data to that of real organs, which helps in accurately identifying cell types and understanding gene regulatory networks within organoids.
One study on brain organoids utilized UMAP (Uniform manifold approximation and projection) to analyse RNA sequencing data at different developmental stages, uncovering the composition of cell subtypes over time. This analysis, combined with data from neonatal electroencephalogram (EEGs) of preterm infants, highlighted the developmental parallels between cortical organoids and fetal human brains, especially in terms of electrophysiological network changes. Furthermore, the creation of the brain and organoid manifold alignment (BOMA) algorithm has facilitated the comparison of gene expression between real brains and organoids, uncovering patterns of gene expression over time and across species, thereby validating the use of brain organoids for neural system research.
Moreover, machine learning extends beyond analysing genetic data. For example, robust support-vector machines (RSVM) algorithms have been used to categorize salivary gland organoids based on changes detected in Raman spectral data during organoid formation. Similarly, a study on cardiac organoids demonstrated that a random forest approach could effectively sort heart cells from non-heart cells in single-cell RNA sequencing data from heart organoids derived from human induced pluripotent stem cells (hiPSCs). This model could also distinguish between heart tissues created from 3D organoids, traditional 2D cultures, and organoids from genetically mutated hiPSC lines, showcasing the nuanced differences in cell types between ventricular and atrial cardiomyocytes.
It should be emphasized that deep learning has significantly contributed development in the field of organoid research by improving the analysis of images and videos captured through microscopy. These visual data sources contain information about organoid development, including changes in morphology, size, and function, to understand their response to environmental changes. The amount of data generated, however, has created a bottleneck in interpreting these experimental results, necessitating the adoption of deep learning techniques for efficient organoid image analysis and tracking. Significant efforts have been made to develop tools that are using deep learning to automate the quantification of changes in organoids, addressing the challenges of manual screening which is both time-consuming and error-prone.
A specific dataset designed for high-throughput organoid image analysis employs Convolutional Neural Networks (CNN) architecture, Residual Network (ResNet), a type of CNN architecture designed to handle very deep networks, both enabling the tracking of organoid growth and morphological changes with high speed and accuracy. This breakthrough demonstrates deep learning's potential to improve organoid research. Open-source tools like MOrgAna combine deep learning with traditional machine learning to analyse brain organoid images, using deep architectures to differentiate organoid pixels from noise, thereby improving upon existing quantification methods.
Applications of Organoid Intelligence
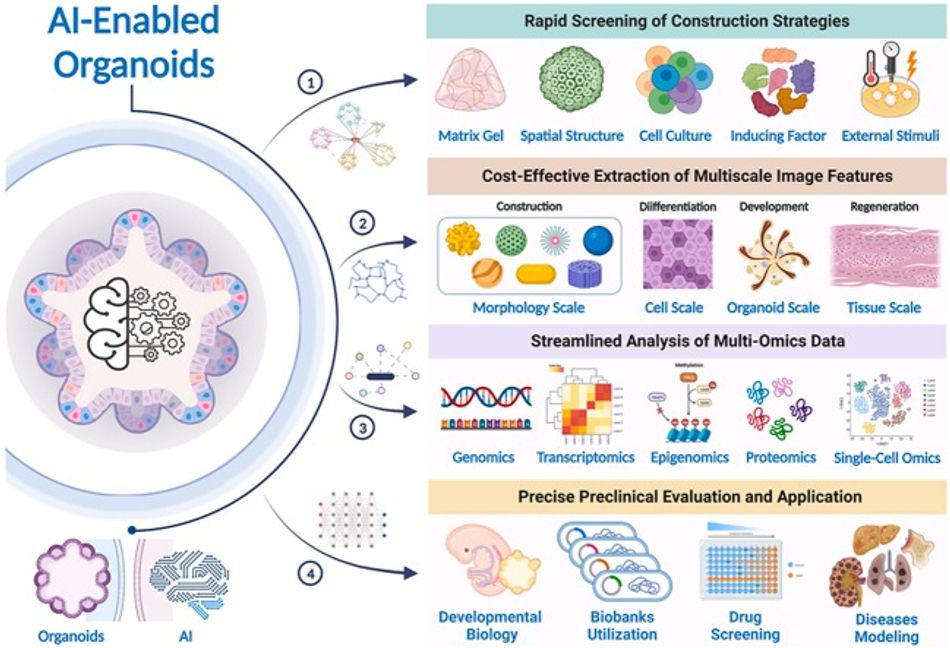
Integrating organoids with AI aims to refine models of human organ functionality and disease, offering a new approach for developing and advancing the drug discovery, diagnostics, and therapeutic innovations. The role of AI is in improving organoid technology across five aspects: swift identification of construction methods, cost-effective analysis of complex image data, efficient multi-omics data interpretation, accurate preclinical assessments, and practical applications. These areas provide a comprehensive view of AI's potential to accelerate and refine the development of AI-improved and supported organoids.
Rapid identification of construction methods is important for selecting the best procedures for organoid creation, while cost-effective analysis of complex image data allows for deeper insights into organoid structure and function from various perspectives. Streamlined interpretation of multi-omics data enables a thorough understanding of organoid biology, encompassing gene expression, proteomics, and metabolomics. Accurate preclinical assessments and applications are essential for predicting AI's real-world clinical utility.
Practical strategies for applying these concepts will maximize AI's impact on developing organoid technology. More specifically, a machine learning framework has been developed to forecast the efficacy of anti-cancer drugs using organoid models, highlighting the predictive biomarkers in cancer treatment optimization. However, current machine learning techniques often face challenges in extracting reliable biomarkers from preclinical models. To address this, a novel network-based strategy utilizing pharmacogenomic data from 3D organoid cultures has been introduced, demonstrating precise drug response predictions in colorectal and bladder cancers. This approach underscores the potential of AI in refining cancer therapies through better biomarker identification.
A notable study introduces IRENE, a transformer-based model that improves multimodal inputs for clinical diagnostics, showing superior performance in diagnosing and predicting clinical outcomes compared to traditional models. This study indicates the growing importance of machine learning in disease modelling and treatment development, poised to significantly impact clinical diagnostics and personalized medicine as our technological capabilities and understanding of organoids and disease processes continue to evolve.
Challenges and Future Directions
The integration of AI with organoid technology presents several ethical considerations and societal implications. One of the primary ethical concerns revolves around the use of human stem cells for creating organoids. This includes questions about consent, especially when cells are derived from embryos or patients who may not be fully aware of the potential future uses of their cells. Societal implications include issues of accessibility and equity. As organoid technologies advance, there's a risk that treatments developed using these cutting-edge methods could be expensive and only available to a privileged few, exacerbating existing healthcare disparities. Moreover, the potential for creating patient-specific organoids for precision medicine raises privacy concerns regarding genetic data and the need for robust data protection measures.
From a technical perspective, several challenges and limitations need to be addressed. One of the main challenges is the complexity of human organs, which are difficult to replicate in full of current organoid technology. While organoids can mimic certain aspects of organ structure and function, they often lack the complete cellular diversity and organ-level functions, such as blood supply and immune system interactions.
Addressing these challenges requires a multidisciplinary approach that combines recent findings in stem cell biology, tissue engineering, computational sciences, and ethics. Future research should focus on improving the fidelity and complexity of organoids to more closely mimic human organs, including the development of vascularized and innervated organoids that can better replicate organ functions.
Conclusion
AI-enabled organoids have the potential to unlock deeper understandings of the biological systems represented by various organoid models. An important part of this advancement is the effective creation and maintenance of organoids, areas where AI, especially through machine learning algorithms, shows considerable promise. AI's ability to process and analyse amounts of omics data provides valuable feedback on organoid functionality and construction techniques. This leads to more effective and higher-quality organoid production, facilitating a smoother progression from experimental research to practical clinical use.
