Bioengineered Skin Grafts that Fit Like a Glove
Columbia University devising a way to grow engineered skin in complex, three-dimensional shapes, making it possible to construct, for example, a seamless “glove” of skin cells that can be easily slipped onto a severely burned hand.
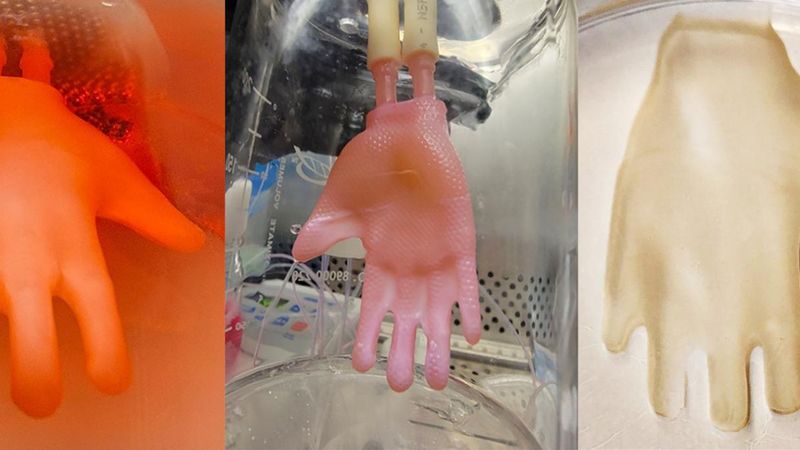
Creating a "glove" of engineered skin begins with a 3D-printed scaffold and ends three weeks later with a construct ready for grafting. Images: Alberto Pappalardo and Hasan Erbil Abaci / Columbia University Vagelos College of Physicians and Surgeons.
If you’ve ever tried giftwrapping an odd-shaped present like a teddy bear, you can appreciate the challenge that surgeons face when grafting artificial skin onto an injured body part. Like wrapping paper, engineered skin comes in flat pieces, which can be difficult and time-consuming to stitch together around an irregularly shaped body part.
Bioengineers at Columbia University appear to have solved this problem by devising a way to grow engineered skin in complex, three-dimensional shapes, making it possible to construct, for example, a seamless “glove” of skin cells that can be easily slipped onto a severely burned hand.
The researchers reported their findings in a paper published Jan. 27 in Science Advances(link is external and opens in a new window).
“Three-dimensional skin constructs that can be transplanted as ‘biological clothing’ would have many advantages,” says lead developer Hasan Erbil Abaci, PhD, assistant professor of dermatology at Columbia University Vagelos College of Physicians and Surgeons. “They would dramatically minimize the need for suturing, reduce the length of surgeries, and improve aesthetic outcomes.”
The current study also revealed that the continuous 3D grafts have better mechanical and functional properties than conventional, pieced-together grafts.
3D scaffolding
The process of creating the new skin grafts begins with a 3D laser scan of the target structure, such as a human hand. Next, a hollow, permeable model of the hand is crafted using computer-aided design and 3D printing. The exterior of the model is then seeded with skin fibroblasts, which generate the skin’s connective tissue, and collagen (a structural protein). Finally, the outside of the mold is coated with a mixture of keratinocytes (cells that comprise most of the outer skin layer, or epidermis) and the inside is perfused with growth media, which support and nourish the developing graft.
Except for the 3D scaffold, the researchers employed the same procedures used to make flat engineered skin and the entire process took the same time, about three weeks.
In a first test of the 3D engineered skin, constructs composed of human skin cells were successfully grafted onto the hind limbs of mice. “It was like putting a pair of shorts on the mice,” Abaci says, “The entire surgery took about 10 minutes.” Four weeks later, the grafts had completely integrated with the surrounding mouse skin, and the mice reacquired full functions of the limb.
Mouse skin heals differently than human skin, so the researchers next plan to test the grafts on larger animals with skin biology that more closely matches that of humans. Clinical trials on humans are likely years away.
Redesigning engineered skin
The 3D grafts are the first major re-design of engineered skin grafts since they were first introduced in the early 1980s. “Engineered skin started with only two cell types, but human skin has around 50 types of cells. Most research had focused on mimicking the cellular components of human skin,” Abaci says. “As a bioengineer, it’s always bothered me that the skin’s geometry was overlooked and grafts have been made with open boundaries, or edges. We know from bioengineering other organs that geometry is an important factor that affects function.”
Abaci and his team realized they could make more lifelike grafts when 3D printers became available and could create three-dimensional scaffolds necessary for making the engineered skin.
“We hypothesized that a 3D fully enclosed shape would more closely mimic our natural skin and be stronger mechanically, and that’s what we found,” Abaci says. “Simply remaining faithful to the continuous geometry of human skin significantly improves the composition, structure, and strength of the graft.”
In the future, Abaci envisions grafts could be custom-made from a patient’s own cells. With only a 4X4 mm skin sample, enough cells can be cultured and multiplied to create enough skin to cover a human hand.
“Another compelling use would be face transplants, where our wearable skin would be integrated with underlying tissues like cartilage, muscle, and bone, offering patients a personalized alternative to cadaver transplants,” Abaci says.
The study is titled “Engineering Edgeless Human Skin with Enhanced Biomechanical Properties.”
The other contributors (all at Columbia University): Alberto Pappalardo, David Alvarez Cespedes, Shuyang Fang, Abigail R. Herschman, Eun Young Jeon, Kristin M. Myers, and Jeffrey W. Kysar.
