Rechargeable Batteries for Greener Solutions
Article 2 of the Power Conversion Series. Rechargeable batteries provide untethered power, enabling convenience, dependability, and mobility. Unplug and recharge and learn how rechargeable battery technology makes life better.

Image: Gogoro
This is the second article in a 5-part series exploring power conversion. The series will provide insight into how the rapid electrification of vehicles and the switch to renewable energy is driving the demand for safe and reliable power conversion and electronic components.
The articles were originally published in an e-magazine, and have been substantially edited by Wevolver to update them and make them available on the Wevolver platform. This series is sponsored by Mouser Electronics, an online distributor of electronic components. Through their sponsorship, Mouser Electronics supports engineers in designing sustainable and efficient applications for a greener future.
Introduction
People don’t want to be tied down. Everyone wants their independence and the freedom to come and go. Something in our DNA makes us want to travel to different places, explore unfamiliar destinations, take in new experiences, or go on summer vacation as we try to unplug and recharge. However, the one thing we can’t unplug from or leave behind is our dependence on technology.
Batteries provide untethered power, enabling convenience, dependability, and mobility—freedom. Environmental responsibility suggests that rechargeable batteries have the same benefits but can save money while reducing waste. In this article, we examine the benefits of rechargeable battery technology.
Batteries
Modern society is in love with technology. We employ it to provide us with a better and more comfortable lifestyle. The technological hardware we use is greatly enabled by the physical phenomena of electrical charge in motion—electricity. Alternating current (AC) – current that moves bidirectional - is useful for sending power over vast distances because of its simplicity in changing voltages through the use of transformers. On the other hand, direct current (DC) is a unidirectional current where electricity moves from a negative terminal in the direction of the positive terminal. One of the unique features of DC is that it can get stored for later usage.
Batteries are electrochemical cells capable of storing and delivering DC. They come in various shapes and sizes, and electrical capacities that get designed around their intended usage. Batteries might get classified as either primary or secondary. Primary batteries are not rechargeable; they are designed for a singular usage, whereas secondary batteries are rechargeable and available to be used repeatedly.
Unplug
Energy storage available in the form of DC batteries offers a significant advantage over AC power. It allows users to be highly mobile. By eliminating the need to be directly connected to an AC power source, batteries enable users untethered freedom to use electronic devices on the go. Unplugging leads to higher productivity, flexibility, and efficiency. It allows users to be untethered from physical locations such as power plants, factories, offices, and homes and be out and about. After all, few of us today want to be tied to an electrical outlet all day.
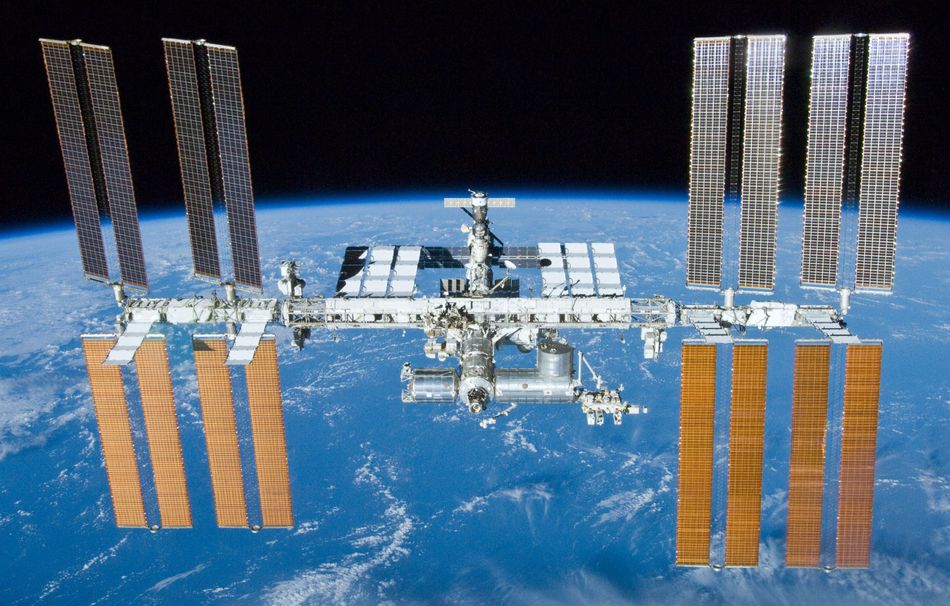
How far out and about one might ask? Well, pretty far. NASA astronauts Christopher J. Cassidy and Robert L. Behnken aboard the International Space Station (ISS), recently worked to replace various DC batteries aboard the station. (Figure 1). NASA reports the astronauts, swapped five aging nickel-hydrogen (NiH2) batteries with two new lithium-ion (Li-Ion) batteries. One more NiH2 battery was swapped for a Li-Ion battery on the Starboard-6 truss structure worksite. Rechargeable batteries allow us to be unplugged even into the vast domains of space.
Recharge
Besides allowing us to go unplugged, secondary batteries are rechargeable. Rechargeable batteries can be recharged repeatedly (Figure 2).
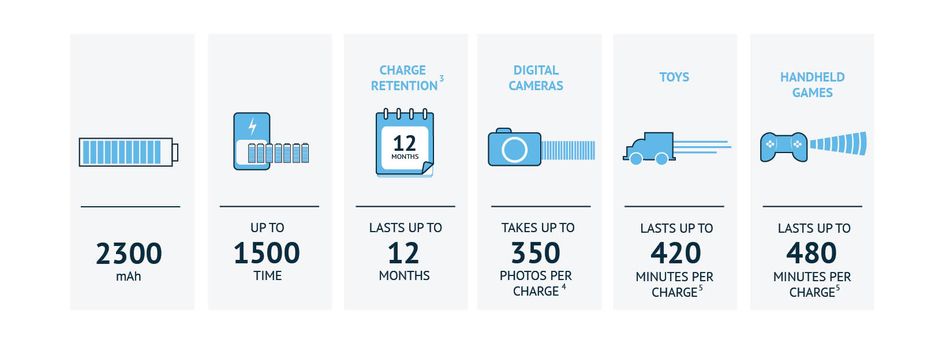 Figure 2: Common rechargeable battery statistics.
Figure 2: Common rechargeable battery statistics.Recently, I returned home from one week’s vacation. I immediately noticed my overgrown lawn because of the heavy rains in my absence. Coming home from work the day after my return, I knew the first thing I had to do was mow. An hour later, I was done, hot and sweaty from the 37˚C temperature ever common to Texas in the summer and full of ant or mite bites up and down my legs. By late evening, these bites drove me insane with the itching and the desire to scratch them, which we all know we are not to do. My only hope was to alleviate the pain was to go to bed and sleep it off. I took two antihistamine diphenhydramine (Benadryl) pills and went to sleep. I slept through the night and woke up refreshed and no longer itching.
It turns out, all I needed was a recharge. As humans, we recognize that when we drain ourselves physically, mentally, and emotionally, periodically, we need to take time to recoup our power and energy. Humans biologically accomplish this through sleeping, a sort of biological recharging. Rechargeable batteries emulate this human capability to get reenergized.
One important ramification of this is that battery costs must be reevaluated over time. In many cases, primary batteries are less costly to purchase. However, when the amount of current consumption is calculated over time, only buying a secondary cell initially and then getting many low-cost recharges out of it will make the economic benefits of rechargeable batteries stand out as very advantageous. The principle of the total cost of ownership (ToC) should be examined based on the specific application context.
Another excellent side benefit of being rechargeable is the impact that this has on environmental issues. We desire to live in a world whereby humankind stewards its resources well and works to mitigate harm to the environment. There is general agreement globally that every business endeavor should commit to and work to achieve initiatives that promote greater environmental responsibility. The ecological impact of a product is often not priced into a ToC calculation but it should be (Figure 3).
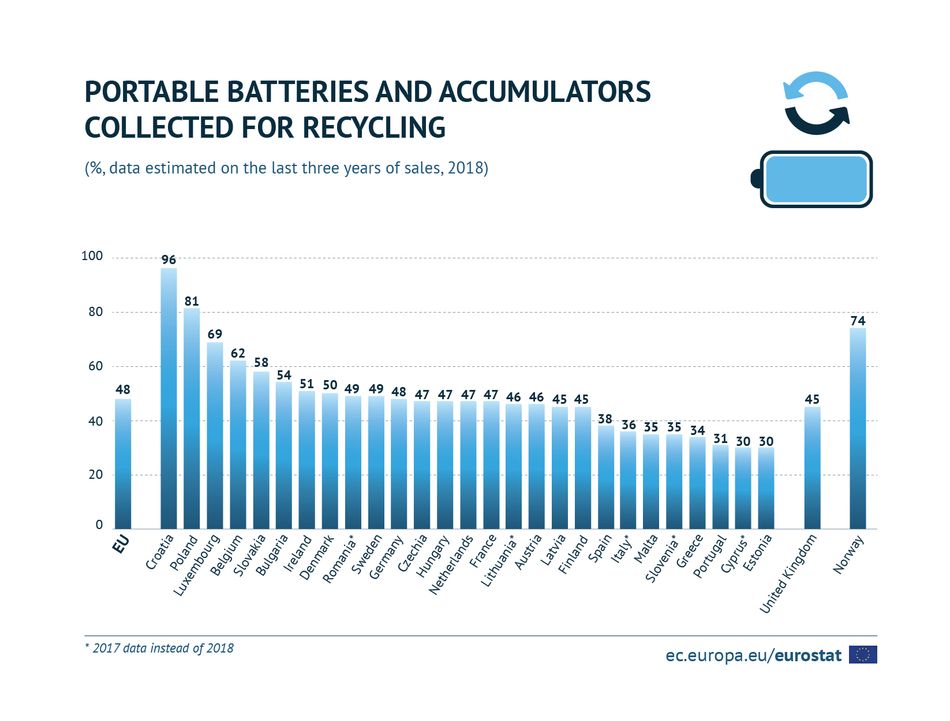 Figure 3: Leading countries for battery recycling.
Figure 3: Leading countries for battery recycling. Because batteries are electrochemical devices, there needs to be an impetus to reduce their resultant chemical waste. Primary units, as mentioned, are suitable for one-time usage. They must be disposed of after they lose their charge. Rechargeable batteries reduce chemical and solid waste.
Capacity
How long can a battery go before it needs to be replaced (primary cell) or recharged (secondary cell)? Battery life is determined by a battery’s capacity to hold an electric charge. This battery capacity gets measured in Ampere hours (Ah). Because rechargeable batteries are electrochemical devices, capacity is limited in a primary aspect based upon how large the physical cell will be. In small devices or portable applications, it can be expressed in milliamp-hours (mAh).
Inherently large loads, items drawing large currents, will discharge a battery quicker. An adjustment factor, named consumption rate, is generally applied to any battery life calculations. Consumption rate helps account for the reality that the battery will ultimately decrease in its ability to deliver current at some point before it gets fully discharged. Mouser Electronics provides a useful battery life calculator for engineers to make quick calculations.
Battery Life = Battery Capacity (Ah) ÷ Load Current (A) x Consumption Rate (0.7 default)
Runtime = 10 x Ampere Hours (Ah) ÷ Load in Watts (W)
Number of Charges
Secondary batteries provide unplugged freedom and the ability to be recharged. But how many times can they get recharged before they break down into failure? The number of recharges available should exceed the expected number of discharges that will occur to be most advantageous. As is the case of the secondary batteries aboard the space station mentioned earlier, there is a limit. These have to be some of the most highly designed cells in usage, yet they too ultimately wear out and need replacement.
Most everyone is familiar with rechargeable batteries in a specific form available for commercial and reusable use in products employed in our everyday lives. Often this takes the form of rechargeable batteries used in cameras, remote controls, flashlights, smoke detectors, remote control toys and hobbies, and clocks. Often what is employed is a standardized rechargeable battery in the AA or AAA category. These types of readily used rechargeable batteries are recognized to be able to be charged to 1,000 (103) times before they wear out.
Capacity Retention
Have you ever pulled out two fresh batteries and plugged them in and found them not ready to function? This effect can be caused by an issue known as capacity retention. You can think of this through the analogy of knowledge you gained in college such as Algebra or Latin. At one time, you knew them well – you were fully charged. But alas, over time, the knowledge has slowly slipped away. You still maintain some, but it is a shell of its former self.
Likewise, batteries do not just sit at 100 percent charge once they reach it. Instead, they generally lose a very minute amount of charge over time, resulting in a shrinkage of their total available charge. In simple terms, this is known as the battery’s shelf-life. How long can it stay in storage before it ultimately trickles away, and its charge becomes worn out? For primary cells, this means they are now useless. For a rechargeable cell, they simply must be recharged, and then they will be fresh.
Common Types
Mouser Electronics provides various batteries and battery technologies from industry-leading manufacturers. Mouser is an authorized distributor for many battery manufacturers, including Panasonic, Phoenix Contact, Power-Sonic, Renata, RRC Power Solutions, Seiko Instruments, Tadiran Batteries, Ultralife, and many more.
Several different types of materials are employed to make rechargeable batteries. Some of the most common types include lead-acid (Pb-acid), Li-Ion, Lithium Iron Phosphate (LiFePO4), Nickel Cadmium (NiCd), Nickel Metal Hydride (NiMH). Let’s quickly take a look at each of these.
Lead-acid
If you own or ride in an automobile, you should be readily familiar with this type of rechargeable battery. Lead-acid batteries are the default type of battery found in most gas combustion automobiles. Lead-acid batteries have a high power to mass performance that makes them suitable for high amperage demand applications. These include turning over an automotive starter that requires lots of energy to turn over.
Li-Ion
They work well with automotive, aerospace, communication, medical, military, and industrial applications. These batteries are commonly found in items such as portable devices, power tools, and electric vehicles (EV). They provide excellent energy density in lightweight packages. They were the subject of much scrutiny when it was observed that incorrect storage could lead to dangerous situations in confined spaces such as airplanes.
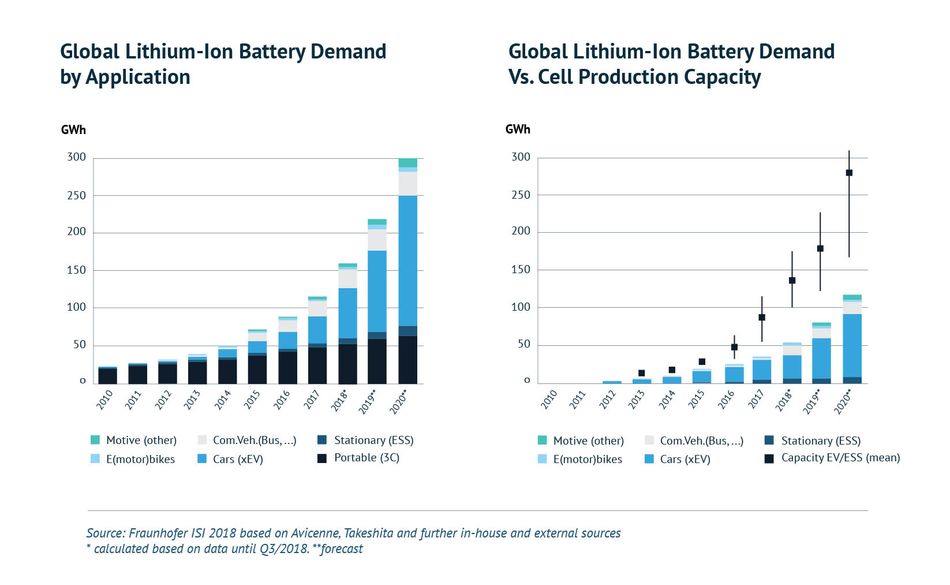 Figure 4: Lithium-Ion Battery demand trends.
Figure 4: Lithium-Ion Battery demand trends.Lithium Iron Phosphate (LiFePO4)
A type of Li-Ion, it offers a longer number of full charge/discharge cycles, known as life cycle. In comparison to a lead-acid battery, Li-Ion batteries have less mass and many times higher life cycle. Suitable applications include medical, solar and wind, mobility, transportation, sports and recreation, and utility.
Nickel Cadmium (NiCd)
The employment of Cadmium brings higher costs relative to nickel-metal or Li-Ion, and the chemical is toxic. NiCd batteries are well-suited for applications that expose the cells to deep discharges such as in harsh environments. NiCd batteries also enjoy the benefit of offering a high-cycle life.
Nickel Metal Hydride (NiMH)
Newer and less toxic than NiCd, Nickel Metal Hydride batteries have good charge capacity. Commonly found at checkout counters around the world at 1.2V, they are excellent in high-current drain applications. They supply a constant voltage level until they are fully discharged, not being susceptible to voltage dropping found in some other types of batteries.
Protecting Li-Ion Batteries and Packs
To ensure long life and reliability, individual battery cells, and battery packs, must be electrically protected. Bourns has been doing just that for decades, while it also continually innovates and expands its portfolio. Bourns’ broad portfolio allows designers to select the right circuit protection component to meet their increasingly complex, demanding, and compact battery pack requirements.
Managing Li-Ion Battery Packs
High-voltage Li-Ion battery packs offer significant advantages such as low weight and high-energy density. However, they require Battery Management Systems (BMS) to operate within safe limits. BMS connects to the Li-Ion battery packs and perform four major functions:
- Monitor voltage, current, temperature, state of charge (SoC), state of health (SoH)
- Balance individual cell voltages for maximum battery pack efficiency
- Protect against overcurrent, overvoltage and over-temperature events
- Communicate via CANbus and/or wireless communication protocols
Components suitable for Battery Management Systems include isolation transformers, signal transformers, power inductors, common mode chokes, TVS diodes, fuses, current sensing resistors, and TBU (transient Blocking Units).
Battery Packs
Mobile devices, including smartphones, tablets, and single-lens digital cameras, primarily employ Li-ion battery packs. Larger physical items such as electric vehicles (EV) along with industrial machinery and robotics also use battery packs.
Battery cells have inherent electrical, environmental, and mechanical challenges. When overcharged or overheated, a battery cell can rupture, combust, or explode. Even if overcharging or overheating does not result in a fire, the battery can still be compromised and can be more susceptible to further damage from physical factors, including vibration, impact, and exposure to heat.
Charge and Temperature
During charging and discharging cycles, battery cells can face overcurrent, overvoltage, and overtemperature conditions. The charging process for Li-ion batteries consists of two phases: constant current and constant voltage. In the constant current charging phase, the charge current is applied to the battery until the voltage limit per cell is reached. Li-ion batteries cannot accept a higher voltage charge than specified without being damaged. The constant voltage phase then begins as the applied current declines to a few percent of the constant charge current. During this time, the maximal cell voltage is applied to the battery. For multi-cell battery packs, a balancing phase occurs between the constant current and constant voltage phases to ensure a consistent charge among cells. In such packs, the voltage applied in the constant voltage stage is the product of the number of cells and the maximal voltage per cell.
Electronic Protection
Protection electronics are employed outside the cell(s) to protect from overcharge, undercharge, and external temperatures. Circuit protection solutions for battery packs comprise several devices, which are crucial design considerations during the charging and discharging of the battery pack.
Two prevalent overvoltage and overcurrent protection methods in cell designs utilize battery management Integrated Circuits (ICs) and Field-Effect Transistors (FETs). Most battery packs, battery cells, and specifically single-cell Li-ion battery pack designs will need a second level of protection. Bourns® Multifuse® Polymer PTC (PPTC) devices or the company’s miniature resettable TCO devices, also known as mini-breakers, are optimal overtemperature protection solutions. Also, dual battery management ICs and FETs provide a two-fold level of overvoltage and overcurrent protection. The protection design can include a Current Sense Resistor (CSR) to monitor the voltage/current.
Enhanced battery performance management that relies on tight and low Temperature Coefficient of Resistance (TCR) can be achieved with current sense resistors to measure drift and resistance accurately. Bourns has a comprehensive line of current sense resistors such as the Bourns CRM2512 Chip Resistors. These are high-power current sense chip resistors with thick film technology. These chip resistors are available with a rating of 2W in a standard 2512 chip format. The CRM2512 with wide resistance range is suitable in power supply circuits, including current sensing and current limiting.
Final thoughts
Well, as I come to the end of this, I need to take action and recharge my batteries. I am off to recharge my laptop and mobile phone back up to full power, unplug and recharge my brain cells before heading home and fighting rush-hour traffic.
This article was originally written by Mouser and Bourns in an e-magazine and substantially edited by the Wevolver team. It's the second article of a 5-part series exploring power conversion. Future articles will dive into power conversion solutions for critical applications such as automotive and renewable energy.
Article 1 explored how designers can make design decisions when working with high-voltage energy storage systems.
Article 2 discussed the potential of rechargeable batteries.
Article 3 examined the method for selecting transformers for Battery Management Systems (BMS).
Article 4 explained the necessity of shunt resistors to deliver accurate Battery Management Systems.
Article 5 provided an overview of innovative transformer solutions for power conversion.
About the sponsor: Mouser
Mouser Electronics is a worldwide leading authorized distributor of semiconductors and electronic components for over 800 industry-leading manufacturers. They specialize in the rapid introduction of new products and technologies for design engineers and buyers. Their extensive product offering includes semiconductors, interconnects, passives, and electromechanical components.