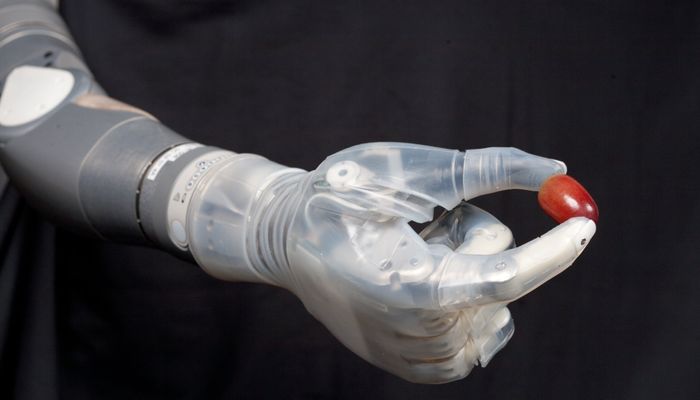
DEKA bionic arm
The DEKA or "Luke" arm is a prosthetic arm that can perform multiple, simultaneous powered movements controlled by electrical signals from electromyogram (EMG) electrodes
Technical Specifications
| Weight | 3.6 |
| 2.5 | |
| 1.3 | |
| Degrees of freedom | 10 Powered |
| Battery | Type |
| Mount | |
| Voltage capacity | |
| Control | Type |
Overview
The bionic arm can be controlled in a variety of ways, depending on the user's preference and ability. Some common control techniques include surface electromyographic electrodes (EMG) and foot-mounted inertial measurement units (IMU). EMG electrodes touch the user's skin and sense electrical activity from muscles in the residual limb that would have moved the natural limb in a desired way. In the case of the IMUs, as the user tilts his or her feet from toe-to-heel or side-to-side, accelerometers in the IMU on the foot send wireless signals to guide the prosthetic arm and hand.
Signals from both EMG and IMU are received by a computer embedded in the prosthesis, which then instructs the motors in the arm to produce simultaneous, complex, multi-directional movements.
Configuration and Technique
The DEKA Arm is available in three configurations, or levels:
1. Radial configuration (RC) - For transradial amputees;
2. Humeral configuration (HC) - For transhumeral amputees
3. Shoulder configuration (SC) - For transhumeral amputees with very short residua, as well as persons with amputations at the shoulder disarticulation and scapulothoracic (forequarter) level.
 Appearance of DEKA Arm Gen 2 and Gen 3 Prototypes: (a) Gen 2 SC Arm on socket/harness without sleeve (left) and with sleeve (right); (b) Gen 3 SC Arm on socket/harness
Appearance of DEKA Arm Gen 2 and Gen 3 Prototypes: (a) Gen 2 SC Arm on socket/harness without sleeve (left) and with sleeve (right); (b) Gen 3 SC Arm on socket/harness
Grips
The prototype supports the use of up to six different pre-programmed grip patterns which provides options for both precision and power grasps. In power grip, the tip of the thumb closes against the tip of the index finger, while the other fingers remain in composite position, forming a loose fist and enabling an object to be held against the palm. Power grip is used to grasp large objects such as bottles, books, and handles. In fine pinch open, the tip of the thumb opposes the index finger and the remaining fingers stay extended and out of the way. Open pinch is used to manipulate and pick up small objects. Fine pinch closed is similar to open tip pinch except that the middle, ring, and fifth digits close in composite position instead of remaining extended.

Six grips of the DEKA Arm (Gen 2 Hand): (a) power, (b) tool, (c) chuck, (d) lateral pinch, (e) fine pinch open, and (f) fine pinch closed.
A chuck grip is similar to a tripod pinch. Chuck grip is used to grasp medium and small size round objects such as bottles, cups, or doorknobs. Lateral pinch is used to grasp flat items such as keys, files, or papers, and writing and eating utensils. In tool grip, the index finger flex to touch the lower part of the thumb, while the middle finger, ring finger, and small fingers close in semi-bent position. This grasp can be used to perform activities requiring a pointer finger, such as typing or playing the piano. If the user actively uses the grasp, the index finger can be flexed to activate a tool such as an electric drill, or utilize a spray bottle, while the remaining fingers remain flexed to stabilize the object.
 Grips with detents: (a) tool grip at first detent, (b) tool grip closed fully, (c) lateral grip at first detent, (d) lateral grip closed fully, (e) closed pinch grip at first detent.
Grips with detents: (a) tool grip at first detent, (b) tool grip closed fully, (c) lateral grip at first detent, (d) lateral grip closed fully, (e) closed pinch grip at first detent.
Powered movement
The SC DEKA Arm has 10 powered degrees of freedom and additional passive degree of freedom .2,3 Powered degrees of freedom include shoulder abduction/adduction; shoulder flexion/extension; humeral internal/external rotation; elbow flexion/extension; forearm pronation/supination; wrist flexion/extension; index finger flexion/extension; middle, ring, and fifth finger flexion/extension; thumb flexion/extension; and thumb abduction/adduction.
At the HC and SC levels, the control scheme has dual modes enabling the user to switch between a “hand mode” of operation (to control movements of the hand and wrist) and an “arm mode” of operation (to control movements of the powered elbow, shoulder, or combined movements, that is, Endpoint Control). In Gen 3, changes have been made to enable availability of up to three movements of the hand and/or wrist in arm mode, if sufficient control inputs are available.
Controls
The arm is controlled by prosthetic movements with a combination of foot controls, myoelectrodes, pneumatic bladders or other commonly available prosthetic input devices. Users work with the prosthetist to identify the appropriate combination of these potential control elements, and did not necessarily use all, or any specific, control elements other than some type of foot control.
IMUs, mounted on a clip that attached to the top of the shoe, utilizes gyroscopes and micro electromechanical systems (MEMS) accelerometers to sense small movements of the foot/ankle in space in relation to a flexibly programmed “zero” position. The user commands motion of the prosthesis by tilting his or her foot (and the IMU) in various directions.
 Three iterations of foot controls: (a) FSR footpad wired to ACI (left) and ACI unit and battery (right); (b) IMU-1 and ACI battery unit worn on shoes (left) and boots (right); (c) IMU-2 on clip (left) and worn on laces (right).
Three iterations of foot controls: (a) FSR footpad wired to ACI (left) and ACI unit and battery (right); (b) IMU-1 and ACI battery unit worn on shoes (left) and boots (right); (c) IMU-2 on clip (left) and worn on laces (right).
These user inputs are received and digitized by one or more ACIs which then transmits these signals to a master control module (MCM). The MCM generates and transmits the arm control commands to the respective electrical module that controls the mechanical prosthetic movements. All control inputs are initially configured by a wireless graphical user interface (called the prosthetist interface (PI)) that run on a personal computer and is available to the clinician, but not designed for the amputee user.
MCM: master control module.
The wireless communication connection between the computer and the MCM, as well as between the foot controls and the Arm, is upgraded during Gen 2 and replaced at the start of Gen 3 with an updated wireless communication connection to increase signal reliability and decrease interruptions in device functioning due to communication losses.
 Gen 2 Master Control Module (left) and battery pack (right) worn on the back.
Gen 2 Master Control Module (left) and battery pack (right) worn on the back.
Power sources
The DEKA Arm is battery powered using a rechargeable lithium-ion battery typically worn in a holster on a belt around the waist or on the back. The control uses a standard 9-V battery that needs replacement when discharged. The size of the external rechargeable battery has been decreased between Gen 2 and Gen 3, and the holster in which it was mounted was redesigned for ease of insertion and removal of the battery. A power-save mode has been developed during Gen 2 and Gen 3 that can engage joint brakes to stop motors from running during inactivity, resulting in longer run-times for batteries in HC and SC Arms.
In Gen 3, an alternating current (AC) adaptor and charging dock replaces the Gen 2 cable system that charged one battery at a time. The Gen 3 charging dock can charge two external batteries simultaneously and is easier to operate with only one hand. The Gen 3 for HC and SC also has the potential to include an internal battery built into the Arm. The internal battery itself is introduced late in the Gen 3 phase. RC level Gen 3 Arms does not have an internal battery. When an internal lithium-ion battery was used, the Arm was turned on and off by a button located in the forearm above the wrist area.
Internal batteries are charged via an AC adapter between an electrical outlet and a charging port on the forearm of the Arm and are expected to offer run time of at least 1 h between charges, depending on intensity of use. In all cases, the internal battery can be augmented by an external battery which was expected to offer run time of at least 6 h between charges, depending upon the intensity of use. The internal ACI battery for IMU-2 is a rechargeable lithium-polymer battery with average run time specifications of a full day between charges. The entire IMU-2 unit is placed on a charging pad which was updated during Gen 3 by adding light emitting diode (LED) signals that indicated when the IMUs were charging.
Results and Discussion
Although many have described the development of multifunctional prosthetic hands and other prosthetic features, there is a paucity of articles describing devices that have been evaluated in clinical studies of persons with amputation. Only a few powered multi function prosthetic hand designs, including the i-Limb products and the Ottobock Michelangelo Hand, have reached the commercial marketplace. Historically, the design and features of devices in the commercial marketplace have not been identified or fully described in the scientific literature and descriptions are only available through technical specification documents produced by the manufacturer after commercialization.
Some prostheses are now available with passive wrist flexion and extension, and many commercially available devices have powered pronation and supination, at this time there are only a few commercially available wrists that incorporate powered flexion and extension. Two commercial wrists have powered wrist flexion: the Centri Hand and wrist which combines wrist flexion and finger flexion, and the hand and wrist supplied by Shanghai Kesheng Prostheses Co. Ltd, that has separate powered wrist flexion. We located only a single published peer-reviewed article describing the engineering design of a 2-DOF prosthetic wrist. However, the Intrinsic Hand supported by DARPA’s Revolutionizing Prosthetics Program (RPP) has a 3-DOF wrist that offers flexion/extension, radial/ulnar deviation as well as 360° rotation. None of the designs reported in the literature, or commercially available, utilize a 2-DOF compound wrist movement like the one available in the DEKA Gen 3 wrist.
The success of DEKA’s optimization efforts will be reported in separate articles describing user and clinician feedback as well as users level of functional performance, dexterity, and skill achieved by these users of the DEKA Arm. The DEKA Arm is still an experimental, pre-production prototype, only available for research use and not currently available for commercial use. US Food and Drug Administration (FDA) approval and commercialization will be needed to enable the fruits of these efforts to reach upper limb amputees.
References
Very short paper introducing the bionic arm. Shows the methods, results, and has a discussion.
This article reports on the features and functionality of the Gen 2 and Gen 3 prototypes discussing weight, cosmesis, grips, powered movements Endpoint, prosthetic controls, prosthetist interface, power sources, user notifications, troubleshooting, and specialized socket features.
This article reports on the overall desirability of the DEKA Arm by prototype and by level of prosthesis, on user-perceived benefits of the DEKA Arm as compared to their current prostheses, and summarizes user concerns about taking the device home.