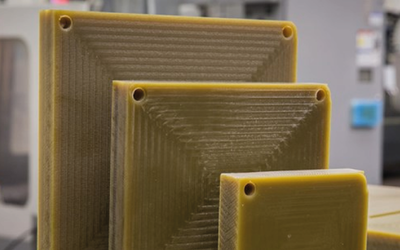
Life Science Grade Thermoplastic Materials
High-performance thermoplastics for medical and bioprocessing applications.
Technical Specifications
| Product Type | Thermoplastic Shapes |
| Body Contact Duration | More than 24 hours |
| Polymer Types | HDPE, PP, PC, PSU, PEI, PPSU, and PEEK |
| Applications | Bioprocessing, Direct and Indirect Body contact applications |
Overview
Life Science Grade (LSG) thermoplastics feature a range of pre-assessed thermoplastic materials for complex bioprocessing and medical applications. Offered by Mitsubishi Chemical Group, LSG materials are bio-compatible according to the USP class VI and ISO 10993 standards, primarily directed for cost-efficient testing and fabrication of medical equipment, especially for short-term body contact applications. The high-performance thermoplastics feature various stock shapes to address the global market needs.
High-Performance Properties for Targeted Applications
The LSG collection of thermoplastic shapes comprises diverse materials for medical and bioprocessing applications. These materials exhibit varying performance properties such as stiffness, temperature resistance, dimensional stability, impact strength, sterilizability, chemical resistance, and gamma radiation resistance. Ranging from Proteus® LSG PP-H to Ketron ® LSG PEEK-CLASSIX, the LSG portfolio accommodates different compliance levels mapping to different performance levels that meet different requirements within the medical and bioprocessing domains.
These materials feature critically robust resistance against different chemicals found in healthcare applications. For instance, Ketron® LSG PEEK-CLASSIX offers the highest resistance against chemical agents like weak dilute acids, strong acids, steam, hot water, and hydrocarbons. Likewise, Proteus® is a non-resistant material to oxidizing chemicals.
The choice of material varies according to the use cases, as they are also designed to cope with sterilization and autoclavability. So, these materials can withstand modern sterilization techniques like dry heat, plasma, steam, EtO (ethylene oxide gas), X-ray, and Gamma radiations.
LSG materials exhibit high transparency to X-rays. The transparency ranges between 71% and 81%, making them suitable for medical imaging applications, radiation therapy, and dosimetry. They also offer high gamma radiation resistance, typically, offering a resistance to radiation index 6.0 and beyond. High resistance makes these materials preferable for sterilization and long-term applications without compromising safety.
Pre-assessed thermoplastic Shapes for Biocompatibility
The range of materials allows device manufacturers to pick the most suitable materials for production. The range of thermoplastics is suited for short-term direct-body applications to bioprocessing and indirect contact applications. These materials offer
High mechanical strength and stiffness
Exceptional chemical resistance
Repeated sterilization compatibility through various methods.
Support up to 30 days of contact with body tissue.
Ketron® LSG materials include:
Ketron® LSG PEEK
Ketron® LSG PEEK Classix (white)
Ketron® LSG FG PEEK
Ketron® LSG CA30 PEEK
Sultron® LSG materials feature a Radel® PPSU base with superior hydrolysis resistance. It is suited for repeated steam autoclaving and hot washing cycles. It can resist common acids and bases over a broad temperature range. It’s quite ductile and available in various colors. Sultron LSG variants include:
Sultron® LSG R5100 PPSU
Sultron® LSG R5500 PPSU
Sultron® LSG FG R5100 PPSU
Sultron® LSG PSU
Duratron® LSG is a Polyetherimide, made from unfilled ULTEM™ PEI resin. The material resin and shape are pre assessed for biocompatibility supporting up to 24 hours of body tissue contact. The material is known for its electrical performance, chemical and hydrolysis resistance, and exceptional thermal properties. These materials are most suited for repeated sterilization applications.
Altron® LSG materials are pre-assessed for biocompatibility for 24-hour body contact. Similar to Duratron® LSG materials, the Altron® offers optimal mechanical and thermal performance. It’s a tough material featuring impact resistance, and dielectric properties. It’s most suited for wetted applications and offers good resistance to most types of sterilization. However, it’s not suitable for steam sterilization.
Proteus® LSG materials feature PP-H and HDPE stock shapes that are pre-assessed for 24 hour body contact applications. These materials offer improved dimensional stability and resistance to steam sterilization, chemical cleaning agents and disinfectants. There is low moisture absorption and high tensile strength that makes them ideal for repeated use. Proteus® LSG materials include:
Proteus® LSG HP PP-H
Proteus® LSG HS PP-H
Proteus® LSG HDPE
References
Recommended Specs
Continue Reading
Submerged arc welding is a standard industrial process wherein an arc is formed between a workpiece and an electrode. It was invented in 1935 by the E. O. Paton Electric Welding Institute in Kyiv, Ukraine as a driving force behind the Second World War. One of the most notable applications of this invention is the T34 military tank.