Cathode Anode: The Basics and Applications
Ever wondered how batteries power your gadgets or how metals are refined to their purest forms? The answers lie in the interplay of cathodes and anodes, two simple yet critical components in electrochemistry. This article will delve into their workings, applications, and challenges.
Introduction
Cathodes and anodes are indispensable components of electrochemical cells and electronic systems. They enable processes like energy storage in rechargeable batteries, metal purification via electrolysis, and image generation in a cathode ray tube. Their influence spans numerous industries, including renewable energy, electronics, and material processing.
Cathodes are electrodes where the reduction reactions occur, i.e., they gain electrons. In contrast, anodes are where oxidation reactions occur, losing electrons. Together, they enable the seamless flow of electric current, powering everything from portable devices to large-scale industrial systems. We gain insight into their pivotal role in electrochemistry by exploring their principles and properties.
The Fundamentals of Cathodes and Anodes
What Are Cathodes and Anodes?
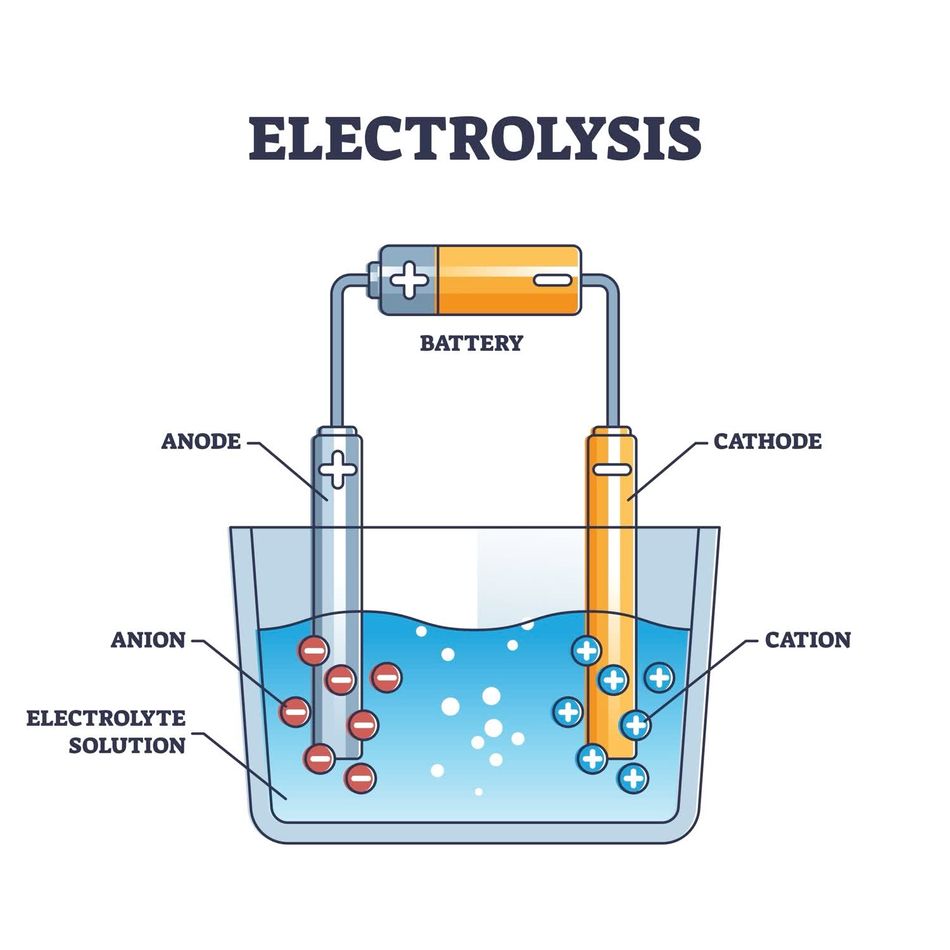
Let’s first start with electrodes. Electrodes are fundamental in systems where chemical energy transforms into electrical energy (or vice versa). They are the backbone of electrochemical cells, acting as conduits for electron transfer and ion movement, enabling processes as diverse as powering a smartphone and purifying metals. Their respective reactions distinguish the cathode and anode:
- Cathode: The positive electrode in a voltaic cell (batteries), where reduction (gain of electrons) occurs.
- Anode: The negative electrode in the same system, where oxidation (loss of electrons) takes place.
But here’s the twist: the labels "cathode" and "anode" aren’t static. Their definitions shift based on the direction of current and the type of system involved. For instance, the cathode is positive in a galvanic cell like a battery. In an electrolytic cell, it’s negative.
This role reversal stems from the need for external energy to drive non-spontaneous reactions, a hallmark of electrochemistry.
Digging Deeper
Let’s connect this to ion movement and current flow. In an electrolyte solution, cations (positive charge ions) move toward the cathode to gain electrons, while anions (negative charge ions) migrate to the anode to release electrons. This movement of ions complements the external flow of electrons in the circuit, ensuring a continuous cycle of energy transfer.
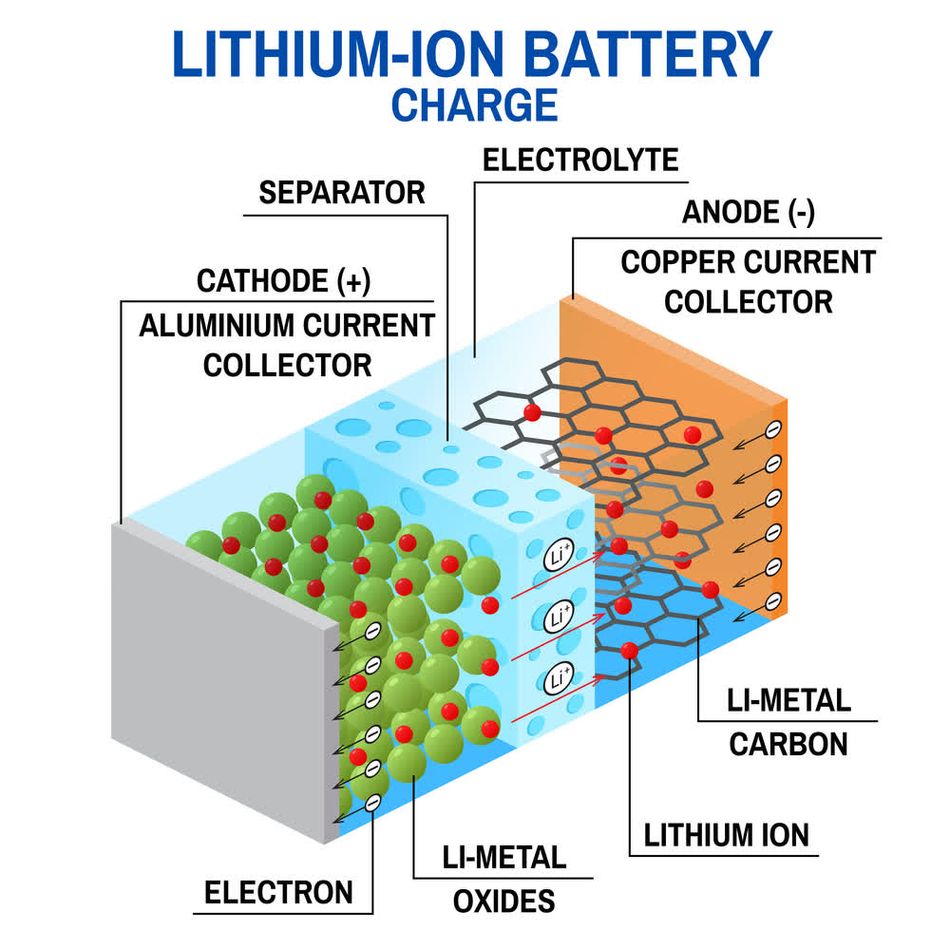
Imagine a lithium-ion battery. Here, lithium ions shuttle between the cathode and anode during charging and discharging cycles. This back-and-forth movement powers everything from your laptop to your electric car. Understanding these movements is crucial not only for appreciating how batteries work but also for advancing their design.
These principles apply across various systems, including batteries, electrolysis setups, and fuel cells.
Mechanism of Electron and Current Flow
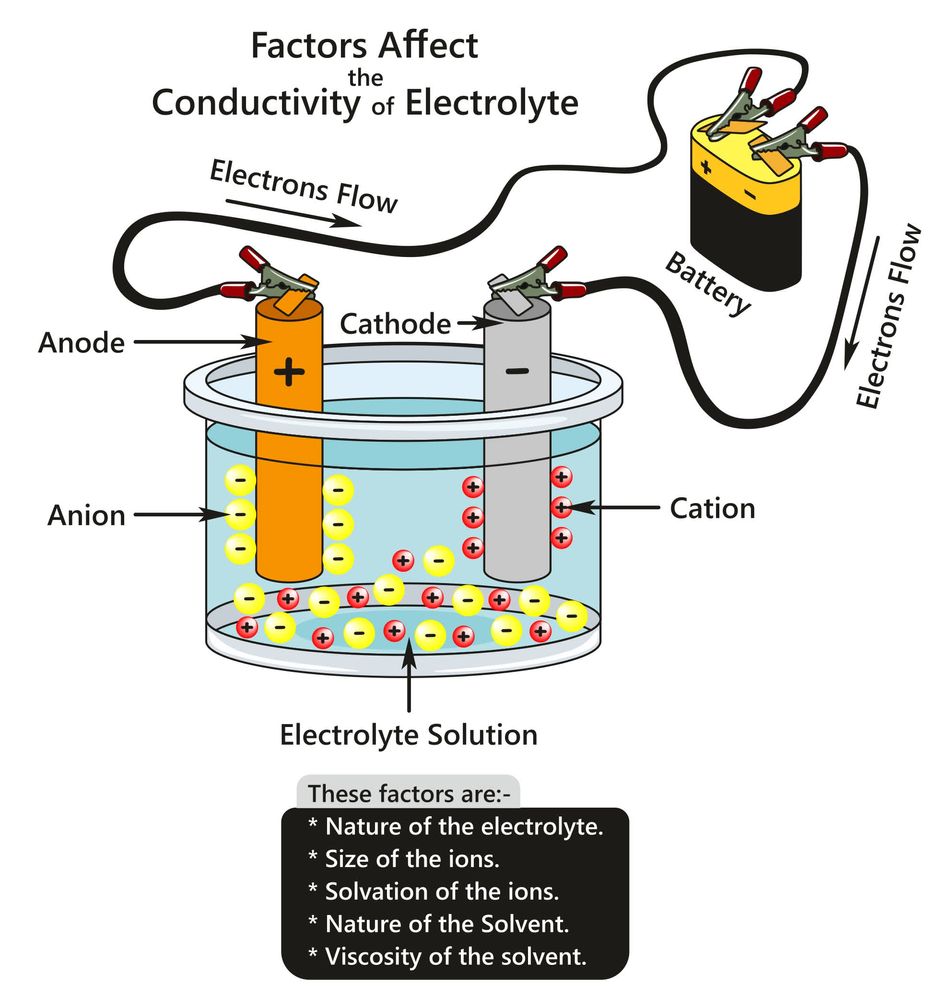
Electron Flow vs. Current Flow
In an electrochemical cell, electrons flow externally from the negative electrode (anode) to the positive electrode (cathode), while inside the system, ions move to maintain charge neutrality. This movement of charged particles creates a balanced, continuous cycle of energy transfer, essential for any electrical device.
Electrical Potential: The Driving Force
The electrical potential difference between the cathode and the anode drives the movement of electrons and ions. This potential is the hallmark of Faraday's contributions to electrochemistry and is a measure of the energy per unit charge available to drive electrons from one electrode to the other. It determines whether a system generates electrical energy (as in a voltaic cell) or consumes it (as in an electrolytic cell) [1].
For example:
- In a galvanic cell, the chemical reaction creates the potential difference, generating electrical energy.
- An external power source applies the potential difference to force non-spontaneous reactions in an electrolytic cell.
A Practical Example: Electrolytic Cell Reactions
Consider the electrolysis of an aqueous sodium chloride solution:
- At the anode (oxidation):
2Cl− → Cl2 + 2e−
Chloride ions lose electrons to form chlorine gas. - At the cathode (reduction):
2H2O + 2e− → H2+ 2OH−
Water molecules gain electrons to produce hydrogen gas and hydroxide ions.
Key Properties and Characteristics
Cathodes and anodes exhibit distinct attributes that are crucial for their roles in an electrochemical cell, directly affecting the efficiency, durability, and functionality of systems like batteries, electrolytic cells, and diodes.
Conductivity: High conductivity is essential for effective electron trnsfer at both electrodes, minimizing energy loss during charge transfer. Techniques such as doping and surface treatments are often employed to enhance this property.
Material Composition
- Cathodes: Frequently made of metal oxides like lithium cobalt oxide (LiCoO₂) in lithium-ion batteries or manganese dioxide (MnO₂) in alkaline batteries, valued for their high energy density and stability.
- Anodes: Commonly crafted from graphite in lithium-ion batteries for its conductivity and long cycle life, or zinc in alkaline batteries for its reactivity and cost-effectiveness.
Material selection balances cost, performance, and lifespan. For instance, lithium cobalt oxide suits high-performance batteries, while manganese dioxide is a reliable, affordable choice for single-use cells.
- Operational Roles: Cathodes facilitate reduction reactions by gaining electrons, while anodes drive oxidation reactions by losing electrons. These processes form the foundation of systems from rechargeable batteries to semiconductors, ensuring efficient energy transfer and chemical transformations.
Table Comparison: Cathode vs Anode
| Property | Cathode | Anode |
| Reaction Type | Reduction (gains electrons) | Oxidation (loses electrons) |
| Polarity (Battery) | Positive charge(+) | Negative charge (-) |
| Polarity (Electrolysis) | Negative charge (-) | Positive charge (+) |
| Common Materials | Lithium cobalt oxide, MnO2 | Graphite, Zinc |
Application-Specific Material Choices
The choice of materials extends beyond conductivity and cost to include factors like reactivity, stability, and compatibility with the electrolyte. For instance:
Lithium cobalt oxide provides high energy density and is ideal for portable devices.
Graphite anodes are crucial for intercalating lithium ions without significant structural degradation.
Applications of Cathodes and Anodes
In Batteries
Batteries are an essential part of modern life, found in everything from smartphones to electric vehicles. At their heart, the cathode and anode facilitate the flow of electrons and ions, allowing energy to be stored and released on demand.
How Do Batteries Work?
Batteries function through a coordinated dance of chemical reactions. During discharge, electrons flow from the anode (negative terminal) to the cathode (positive terminal) via an external circuit, powering your device. Simultaneously, ions travel through an electrolyte inside the battery to maintain charge balance.
Here’s the fascinating part: during charging, the process reverses. An external energy source drives electrons back from the cathode to the anode, restoring the chemical potential for future use. These reactions are governed by oxidation (at the anode) and reduction (at the cathode), highlighting the importance of redox reactions in energy storage.
Battery Types and Their Electrode Materials
- Lithium-Ion Batteries: These are the gold standard for portable electronics and electric vehicles [6].
- Cathode: Usually made of lithium cobalt oxide (LiCoO₂) or similar materials that offer high energy density.
- Anode: Typically graphite, a material that can accommodate lithium ions without significant structural changes.
These batteries are prized for their efficiency and longevity but face challenges like material scarcity and cathode degradation over repeated charge cycles.
- Lead-Acid Batteries: One of the oldest battery technologies still in use, these are commonly found in car engines and backup power systems.
- Cathode: Lead dioxide (PbO₂).
- Anode: Spongy lead (Pb).
Recommended Readings: NiMH vs Lithium Ion Batteries: A Comprehensive Comparison for Engineers
Suggested Readings: Beyond lithium: Sodium-based batteries may power the future
In Electrolysis and Electroplating
While batteries store and release energy, electrolysis uses electrical energy to drive chemical reactions. This process is pivotal in industries ranging from water purification to metal refining, and once again, cathodes and anodes play starring roles.
How Electrolysis Works
Electrolysis involves applying an external voltage to a solution or molten compound, forcing a redox reaction to occur. Here’s how the electrodes work in this context:
- Cathode (Negative Terminal): This is where reduction takes place. Electrons from the external circuit flow into the cathode, allowing positively charged ions in the solution to gain electrons and form neutral substances. For example, hydrogen gas is produced at the cathode in water splitting.
- Anode (Positive Terminal): Here, oxidation occurs. Negatively charged ions lose electrons, often producing gases like oxygen or enabling metal dissolution into the electrolyte.
A classic example is water electrolysis, where hydrogen is produced at the cathode and oxygen at the anode. This process holds immense potential for sustainable energy solutions, as hydrogen can be used as a clean fuel.
Electroplating: Adding Value, Preventing Corrosion
Another fascinating application of cathodes and anodes is in electroplating, where a thin layer of metal is deposited onto a surface to enhance its properties. This process is key to industries like jewelry making, aerospace, and electronics.
Here’s how it works:
- Cathode: The object to be plated, such as a ring or a machine part, acts as the cathode.
- Anode: The source of the plating material, such as a gold or silver bar, is the anode.
During electroplating, a redox reaction occurs where the anode dissolves into the electrolyte, releasing metal ions deposited onto the cathode. This improves the object’s appearance and protects it from corrosion and wear [2][3].
Examples of Electroplating and Purification Processes
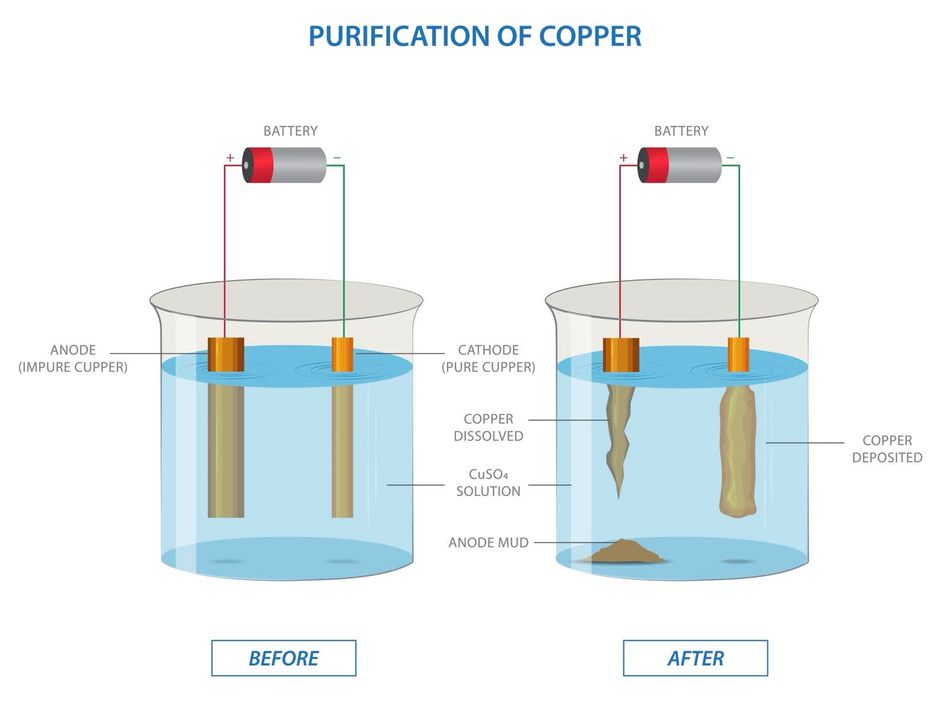
- Electroplating [4][5]: Used to coat objects with a thin layer of metal. For example:
- Cathode: The object to be plated (e.g., jewelry).
- Anode: The metal being deposited (e.g., gold or silver).
- Purification: Used to purify metals like copper:
- Cathode: Pure copper.
- Anode: Impure copper.
Comparison Table: Roles of Cathodes and Anodes in Applications
| Application | Cathode Role | Anode Role |
| Electroplating | Object to be plated | Metal source for deposition |
| Purification | Site for purified metal | Source of impure metal |
| Electrolysis (General) | Reduction reaction (gain of electrons) | Oxidation reaction (loss of electrons) |
Suggested Readings: New Smart Charger May Pave the Way for More EVs
Challenges and Misconceptions
Common Misunderstandings
Despite their importance, cathodes and anodes are often misunderstood. Here are some common misconceptions and practical challenges associated with them:
Misunderstandings
- Polarity Confusion: One common source of confusion is the changing polarity of cathodes and anodes across applications. For instance, in batteries, the cathode is positive, but in electrolysis, it’s negative. Understanding this difference is crucial for accurate system design.
- Electron Flow Direction: Regardless of the application, electrons always flow from the anode to the cathode in the external circuit. This unchanging rule helps simplify analysis.
Practical Challenges
- Material Degradation: In batteries, both cathodes and anodes degrade over time due to repeated cycling, reducing efficiency and capacity. Lithium-ion batteries, for example, experience cathode material breakdown, which affects their lifespan.
- Resource Limitations: Critical materials like cobalt are expensive and scarce. Researchers are exploring alternatives, but these often come with trade-offs in performance or stability.
Solutions and Innovations
Thankfully, advancements in material science are addressing many of these challenges:
- Improved Electrode Materials: Researchers are developing solid-state electrolytes and composite materials that enhance conductivity, stability, and safety.
- Protective Coatings: Coatings like graphene can reduce corrosion and improve electrode longevity.
- Sustainability Efforts: Recycling programs aim to reclaim valuable materials like lithium and cobalt, reducing reliance on mining and minimizing environmental impact.
Suggested Readings: Electric aviation: Batteries that stay strong for the flight duration
Suggested Readings: How Much of the IOT Battery Capacity is Actually Used
Why Understanding Cathodes and Anodes Matters
From powering our devices to enabling advanced industrial processes, cathodes and anodes are central to modern life. By driving redox reactions, they enable energy transformation, protect valuable surfaces from corrosion, and create the foundation for sustainable technologies .
As we innovate and improve, these unsung heroes will continue to shape the technologies of tomorrow, making our lives more efficient, sustainable, and connected.
Frequently Asked Questions
1. What Is the Difference Between a Cathode and an Anode?
Cathodes and anodes serve different roles in electrochemical systems based on the type of reaction they facilitate.
Cathode: The site of reduction, where the electrode gains electrons.
Anode: The site of oxidation, where the electrode loses electrons.
The polarity of each also varies:
In batteries, the cathode is positive (+), and the anode is negative (-).
In electrolysis, the cathode is negative (-), and the anode is positive (+).
Common materials also differ: cathodes often use materials like lithium cobalt oxide or manganese dioxide, while anodes rely on graphite or zinc.
2. Why Do Cathodes and Anodes Have Different Polarities in Batteries and Electrolysis?
The polarity of cathodes and anodes changes because the flow of electrons and the purpose of the system differ:
In Batteries: The cathode is positive (+) because it accepts electrons during discharge, while the anode is negative (-) as it donates electrons.
In Electrolysis: The cathode is negative (-) because it provides electrons for reduction, and the anode is positive (+) because it removes electrons during oxidation.
This distinction reflects whether the system is generating energy or driving a chemical reaction using external energy.
3. What Materials Are Commonly Used for Cathodes and Anodes?
The materials selected for cathodes and anodes depend on the application and its requirements. For instance:
In Batteries: Cathodes typically use lithium cobalt oxide or manganese dioxide, while anodes are often made of graphite.
In Electrolysis: Cathodes can be copper or silver, and anodes might be zinc or graphite.
In Electroplating: Cathodes are the objects being plated (e.g., jewelry), and anodes serve as the metal source for plating, like gold or silver.
These material choices ensure optimal performance for energy storage, chemical transformation, or decorative purposes.
References
[1] Wikipedia. Faraday’s Law of Electrolysis. Link.
[2] Springer Nature Link. Premature Failure of Submarine Well Fluid Lines: A Case Study. Link.
[3] Science Direct. Risk analysis on corrosion of submarine oil and gas pipelines based on hybrid Bayesian network. Link
[4] ScienceDirect. Electroplating in the modern era, improvements and challenges: A review. Link.
[5] Ganoksin.Electroplating Jewellery. Link.
[6] ScienceDirect. A review on the lithium-ion battery problems used in electric vehicles. Link
Table of Contents
IntroductionThe Fundamentals of Cathodes and AnodesWhat Are Cathodes and Anodes?Digging DeeperMechanism of Electron and Current FlowElectron Flow vs. Current FlowElectrical Potential: The Driving ForceFor example:A Practical Example: Electrolytic Cell ReactionsKey Properties and CharacteristicsMaterial CompositionApplications of Cathodes and AnodesIn BatteriesIn Electrolysis and ElectroplatingExamples of Electroplating and Purification ProcessesChallenges and MisconceptionsCommon MisunderstandingsWhy Understanding Cathodes and Anodes MattersFrequently Asked QuestionsReferences
