Switchable hydrocarbons, easily synthesized, could offer single-molecule components for future electronic systems
An oligomeric [8]annulene-based material has been shown to be suitable for creating single-molecule components including logic elements, switches, and gates — and could be one of the ways Moore's Law is kept marching in the semiconductor industry.
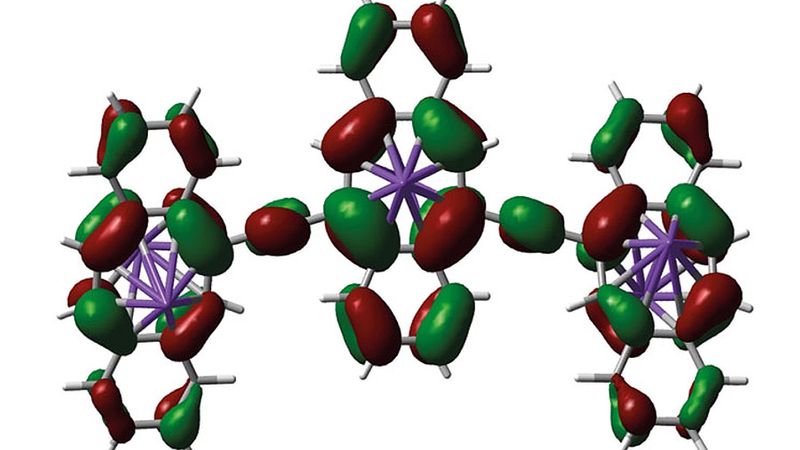
This artist's impression shows the structure of a material which could, one day, replace traditional components in integrated circuits.
The march of Moore’s Law — the observation by Intel co-founder Gordon Moore that the number of transistors in a leading-edge integrated circuit trends towards a doubling every 18 months, since turned into a must-hit target by the semiconductor industry — has been faltering of late, as physical limits on the nanometer-scale components start to bite.
As a result, there’s considerable interest in finding alternatives to traditional silicone — and a team from Lund University and the University of Copenhagen have uncovered an interesting direction for future work, by developing electromechanically-switchable hydrocarbons which could form the basis of single-molecule logic gates, switches, and other components — dramatically reducing the size of future computing systems.
Stable and reversible
The core idea: Using hydrocarbon as a basis for components which can be flipped between two or more states via topological switching. It’s not a new concept, but one which has previously run into a series of roadblocks towards its development — issues which the team, including first author Magdalena Tasić, claims to have resolved through the development of a material which is stable and reversible yet still responsive to electromechanical switching and which is, to its largest credit, straightforward to synthesize.
The creation in question: Oligomeric dibenzo[a,e]cyclooctatetranes (dbCOTs) connected by alkynyl spacers at pseudo-conjugated 5,12-positions of the [8]annulene core. Injecting electrons into an [8]annulene drives a shift in its shape — from a folded tub-shape while in its ground state to a flat aromatic structure in its reduced state.
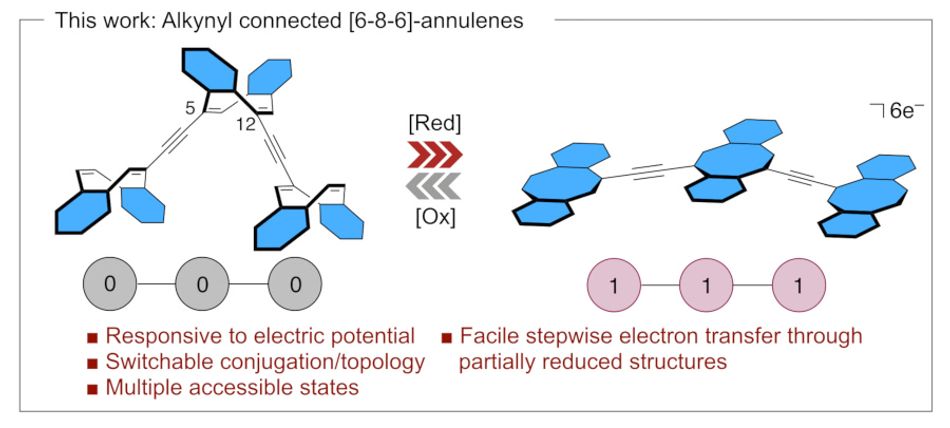
Compared to previous efforts, the team’s pseudo-conjugated dbCOT-oligomers are claimed to offer stable switching over numerous two-electron reduction-oxidation (redox) cycles. At the same time, the design is structurally simple — and synthetically accessible, requiring just three steps to go from a known triflate to the simplest oligomer in the work and six to the longest.
“To our knowledge,” the team writes, "this structure represents the longest well-defined COT-based oligomer reported. The stepwise synthetic approach is attractive as it can, in principle, be used either bi- or uni-directionally to assemble well-defined oligomers of arbitrary length."
Single-molecule switches
In demonstrations of the full redox cycles for all oligomers, the team proved their creations were stable “for days” at room temperature without any measurable degradation while complete redox cycles could be carried out even in solution chemistry — a notably challenging environment. Better still, donation and acceptance of electrons were both fast processes — to the point where electron donors and drains are likely to be the bottleneck in the system.
In terms of practical usage, the team’s work went a stage further: In testing consecutive electrochemically-driven redox cycles, the researchers were able to demonstrate reliable electron acceptance from a surface — “essential,” they note, “for more advanced applications and integration.”
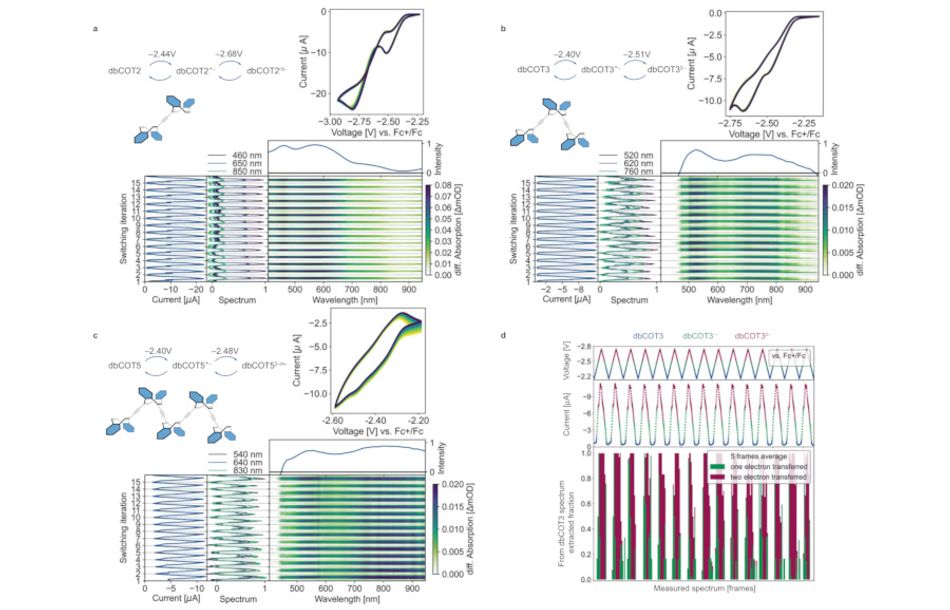
During these tests, the devices showed three distinct “species” — a ground state and two distinguishable reduced states — which could be read out optically, forming the basis of a usable logic device. Further testing showed successful electron injection from a terminal unit to a central unit then on to a second terminal unit — capturing, in the researchers’ words, “the elements of an integrated switch or gate in action” in a single molecule.
The team’s work isn’t quite ready to replace modern electronics just yet, of course, but it would appear to from a sound basis for future work. In particular, the system’s development from a set of basic design principles will make further adaption and refinement easier.
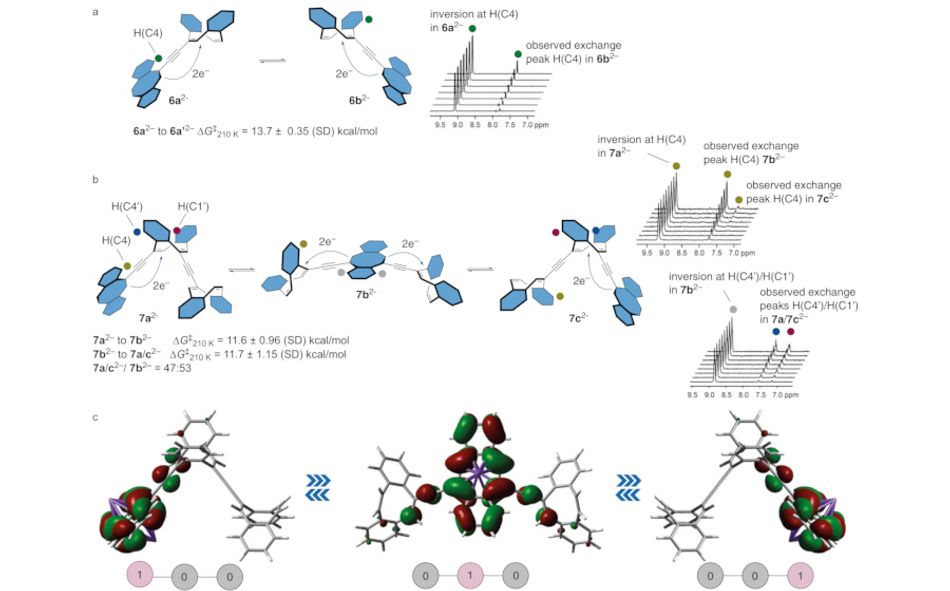
“We anticipate that the presented structures will inspire new entries to shape-shifting materials,” the team concludes, “and provide a leap towards the intriguing possibility of implementation of [8]annulenes as single-molecule logical gates.”
The researchers’ paper has been published in the journal Nature Communications under open-access terms.
Reference
Magdalena Tasić, Jakov Ivković, Göran Carlström, Michaela Melcher, Paolo Bollella, Jesper Bendix, Lo Gorton, Petter Persson, Jens Uhlig, and Daniel Strand: Electro-mechanically switchable hydrocarbons based on [8]annulenes, Nat. Commun. 13. DOI 10.1038/s41467-022-28384-8
